Welcome to the Open Learning Initiative Introduction to Anatomy and
Physiology course!
We are happy that you have decided to introduce yourself to this important field, and we
hope that your learning experience will be an enriching and enjoyable one.
The purpose of this introductory section is to prepare you conceptually and technically
for this course. We will start with a short orientation to the course, including some
learning strategies that will explain a little about how the course works and give you
some pointers on how to use the material most efficiently.
We will then discuss what Anatomy and Physiology is all about—the "Big Picture" of
Anatomy and Physiology—and look at how the major themes discussed in the Big Picture tie
in to the material presented in the course.
Orientation to Anatomy and Physiology
The Introduction to Anatomy and Physiology is a preparatory course that will
introduce you to basic terms and concepts and provide a foundation for your
future study in this discipline.
This course is primarily intended for community college students who need Anatomy
and Physiology knowledge for their selected discipline, and for students who are
planning on entering an allied health program (such as nursing) that requires
prerequisites in Anatomy and Physiology.
Prerequisite knowledge needed to succeed in this course:
- Understanding of the Scientific Method.
- Introductory, college-level Biology and Chemistry.
- Basic math and language skills.
- Study skills.
The course is designed to be offered in a hybrid format to allow you the full
affordances of the online learning environment and the expertise and support of
your instructor.
Your instructor is your first line of support. He or she will be available to you, in
the same way as in your traditional courses. Ask questions in class, or submit
questions and comments through the system. You can e-mail your professor
directly, or provide feedback within the My Response activities provided
throughout the course material.
If you have technical difficulty, you can press the Help button at the top of
any page's browser window. This will open a web form where you can type your
questions or comments and send it to the OLI help desk. Asking for help this way
will send several pieces of contextual information with the message (such as the
course page, your browser, your computer platform) that will help our technical
team diagnose your issue quickly.
If you do submit a question or report an error, please provide as much detail as
possible about the error.
Overall Course Structure
OLI Anatomy and Physiology is not your typical course.
Our goal is for you to work through the course materials online in the way that
is most efficient given your prior knowledge.
While you may have more flexibility than you do in a traditional course, you will also have more
responsibility for your own learning. You will need to:
- Plan how to work through each unit.
- Determine how to use the various features of the course to help you learn.
- Decide when you need to seek additional support.
Each unit in this course has features designed to support you as an independent learner and consists of
the following:
Learning Objectives: Found at the top of each page, these will
help prepare you for what you are about to learn and help check your
understanding of the material on each page.
Explanatory Content: This is the
informational introduction of the basic structures learned in each chapter. It
consists of short passages of text with information, examples, images, and
explanations.
Activities: Activities such as 'Learn By Doing', 'Walkthroughs'
and 'Did I Get This?' These are the most important aspects of the course.
Different types of activities are interspersed throughout the course that will
help you build or test your mastery of the learning objectives.
End of Unit
Quizzes: Taking these quizzes at the end of every unit will assess your mastery
of the learning objectives identified for that unit.
Vocabulary/Terms: There are
many important vocabulary terms throughout the course. Where appropropriate,
audio pronunciation is provided for the terms, as well as definitions in context
that will pop up if you hover over the word.
What is a Big Picture?
One goal of all OLI courses is to promote coherence by teaching students how the discrete
skills they are learning fit together in a meaningful big picture of the domain. The Big
Picture gives students an organizational structure through which they learn the
material.
The Big Picture explains why the material in a course is being covered, as well as how the
material is related or organized. The Big Picture illustrates why one might want to
invest time in learning this material, and what it can do, in a way that students
entering the course will easily understand.
Why Study Anatomy and Physiology?
You probably have a general understanding of how your body works, but to truly understand
the intricate functions of the human body and dispel many misconceptions that you have
learned about your body over the years, you must approach the study of the body in an
organized way.
This course will help you understand those intricacies and attack misconceptions head-on. This
course will expose you to the complex levels of organization taking place inside
the body and provide you with the information you need to delve deeply into the
specific aspects of the body systems. This will prepare you for the more
complex topics you will encounter in your future courses.
There is some agreement among professionals about how to do this, and what information
must be common across all Anatomy and Physiology courses. This is presented as the Big
Picture in Anatomy and Physiology.
Big Picture Ideas in Anatomy and Physiology
Big Picture, Big Ideas, core principles, are all ways to describe the necessary concepts
that make up a discipline. For Anatomy and Physiology, many research studies have been
conducted by various groups to determine what are the Big Ideas in this discipline. In
2007, Joel Michael and his colleagues compiled a list of Big Ideas in
Anatomy and Physiology and then went on to test these ideas with several
comprehensive surveys of professionals and educators in the field.
They determined that the “Big Ideas in Physiology” are:
- Living organisms are causal mechanisms
whose functions are to be understood by applications of the laws of
physics and chemistry.
-
The cell is the basic unit of
life.
- Life requires information flow within
and between cells and between the environment and the organism.
- Living organisms must obtain matter and energy from the external world. This matter
and energy must be transformed and transferred
in varied ways to build the organism and to perform work.
-
Homeostasis (and “stability” in a more
general sense) maintains the internal environment in a more or less constant state
compatible with life.
- Understanding the behavior of the organism requires understanding the relationship
between structure and function (at
each and every level of organization).
- Living organisms carry out functions at many different levels of organization simultaneously.
- All life exists within an ecosystem made up of
the physiochemical and biological worlds.
-
Evolution provides a scientific
explanation for the history of life on Earth and the mechanisms by which changes to
life have occurred.
This course has taken these Big Ideas and used them to structure the material of the
course. We will explain this further on the next page.
Our intention is for you to begin to think and speak in the language of the domain while
integrating the knowledge you gain about anatomy to support explanations of
physiological phenomenon. The course focuses on a few themes derived from the Big Ideas,
that when taken together, provide a full view of what the human body is capable of and
the exciting processes going on inside of it. The themes are:
-
Structure and function of the body,
and the connection between the two.
-
Homeostasis, the body’s natural
tendency to maintain a stable internal environment.
-
Levels of Organization, the major
levels of organization in the human organism from the chemical and cellular levels
to the tissues, organs and organ systems.
-
Integration of Systems, concerning
which systems are subsets of larger systems, and how they function together in
harmony and conflict.
You can see how these themes directly relate to the Big Ideas. As these themes are used
to describe the inner workings of each of the body’s organ systems, those can be categorized
into the specific vital functions for human life. The vital functions provide the
context for the whole body, and how each organ system plays a role in keeping us alive.
So, the information provided for each of the organ systems is organized according to
those functions that are essential to the survival of the human body. The vital
functions for human life are:
- Exchange of substances and information with the environment
- Transport within the body
- Structure, support, and movement
- Control and regulation
- Growth and reproduction
All multicellular organisms need these vital functions to operate properly in order to
survive. In addition to understanding the Themes and Vital Functions, knowing body
planes and directional terms will also help you in your quest for Anatomy and Physiology
mastery.
Body Planes and Directional Terms
Those in the health professions must speak the same language with regard to locating and
identifying specific body parts and organs. Body planes and directional terms are part
of this common language. The imaginary vertical and horizontal planes run through the
body, essentially cutting it into parts. You will be introduced to this new
“language” and given opportunities to practice using it in context so that you become
comfortable locating and describing all organs and parts in the body and in relation to
each other. Everything that you learn after body planes and directional terms will be
referring to this terminology to help you visualize, identify, and locate anatomical
structures.
Body Systems
You will be first introduced to all of the body systems in this introductory unit. In the
units that follow, with the exception of Levels of Organization, and Homeostasis, you
will learn and explore each body system in-depth. The order in which you learn each
system will be determined by your instructor, but the aspects of each system will be
similarly described according to the Big Picture themes.
Everyone has a body and, by adulthood, a general understanding of how it works. But to
truly understand the intricate functions of the human body—and the problems that occur
when something goes wrong—you must approach the study of the body in an organized way.
This course will help you understand the functions of the human body. The course will
discuss the details of many complex functional systems, but will also look at how all of
these systems work in harmony to keep you healthy. As you move through this course, you
should keep four main themes in mind: structure and function, homeostasis, levels of
organization, and integration of systems.
Structure and Function
The first theme is the connection between structure and function. You will be
studying both , which focuses on the body’s structures, and , which focuses on the body’s functions. In fact, it is virtually
impossible to study one without the other, because function relies so completely
upon structure. For example, the structure of the bones in the skeletal system
provides the support necessary for the function of walking upright. The vocal
cords would not be able to fulfill their function—the production of sound—if
their structure were disrupted. The large surface area of the small intestine
allows it to efficiently perform its primary function: absorbing nutrients from
food. And the list goes on.
Homeostasis
The second theme will be , or the body’s natural tendency to maintain a relatively stable
internal environment. Most of the body’s functions are driven by homeostasis.
Homeostasis occurs at all different levels. For example, body temperature is
regulated around 98.6, a temperature that is optimal for cell function and
organism function. To maintain this temperature, we sweat to cool down on a hot
day and we shiver to increase temperature when we are cold. Other variables,
like blood pressure, blood pH, blood calcium concentrations are similarly
maintained within a narrow range that is optimal for human health. Many diseases
occur because of disruptions in homeostasis.
Levels of Organization
The third theme will be the hierarchical organization of the parts of the body.
You can think of the body's parts as being organized into a hierarchy of levels.
Your body, like all things in the physical world, is built from
chemical building blocks. The smallest of these building blocks
are atoms of elements, which combine to form bigger and more complicated
structures called molecules. These molecules, such as water,
proteins, carbohydrates (glucose), and lipids are used to build
cells, the smallest unit of structure capable of carrying out
all life processes. Groups of related cells that work together to perform
specific functions make up , and tissues that work together form organs. Organs do not
work independently; they are organized into organ systems that
complete more complex tasks.
The digestive system, for example, includes the mouth, stomach, intestines, and
many other organs—all of which are integral to proper functioning of the system
as a whole. The organ systems work together to support life in the entire
organism—in this case, a human being.
Understanding this hierarchy is important because disruptions might occur at any
level. For example, a depletion of calcium atoms from the body can lead to weak
bones. Or a single mutation in a DNA molecule can lead to organ dysfunction,
such as the disturbed lung function found in individuals with cystic
fibrosis.
Integration of Systems
Finally, each section of the course will discuss the integration of all the
body’s systems. In order to carry out its functions, every organ system relies
on the healthy functioning of other systems. When these systems all work
together, the organism thrives. A breakdown in one system can cause failures in
other systems as well.
In this section, you will be introduced to the major organ systems of the body. To put
these systems in context, we will first discuss vital functions of life.
Within any organism, there are a multitude of functions taking place at any given time.
Humans, for example, can breathe, talk, digest food, process visual images, and move
their bodies all at the same time. While all of these activities are important, some are
essential to the survival of the human body itself. They are - processes or actions of the body on which life is directly dependent.
You will examine four main vital functions in this course: exchange
with the environment; transport within the body; structure, support, and movement; and
control and regulation.
For human life, there are several vital functions.
So you now know that all multicellular organisms need to do the following in order to
survive:
- Exchange with the environment
- Transport fluids and material throughout the body
- Provide structure, support, protection and movement
- Regulate and control processes
So what does this mean? What does this involve? How does the human body do these things?
Try answering the questions below to begin broadly thinking about bodily function within
these categories and how they are linked to one of the primary organ systems.
Exchange with the Environment
An organism constantly interacts with its environment. In order to survive, the
human body must obtain food, water, and oxygen from the world around it. The
human body must also rid itself of wastes before they build up to toxic levels.
Three organ systems are primarily responsible for exchange with the environment.
The
system brings food and water into the body and eliminates solid
wastes. The
system brings in oxygen and removes carbon dioxide.
Fluids and Transport within the Body
Single-celled organisms can absorb nutrients and oxygen directly from the
environment into the cells, where they are used to support basic cell functions.
Waste products are excreted from these single cells in a similar fashion. In
multi-celled organisms like humans, however, most cells are not exposed directly
to the outside environment. Instead, body cells rely on organ systems to
transport molecules throughout the body. Three main body systems, the
system, the
system, and the
system, take care of this vital bodily function. The urinary system
filters out and eliminates the waste products of metabolism. The cardiovascular
and lymphatic systems also participate in the function of immunity,
to help defend the body's cells from foreign organisms that may enter the body
tissues or fluids.
Structure, Support, Protection, and Movement
For the organs of the human body to function, they must be protected from
potentially damaging substances in the environment. One level of defense is
provided by the
system, made up of the skin, hair, and nails. This system prevents
many potentially harmful irritants from entering the body. Eyelashes, for
example, help keep sand or other items out of the eyes, where they could
potentially cause serious damage, and the skin prevents most pathogens
(disease-causing microorganisms) from entering the body and destroying healthy
body cells. Certain parts of the
system, such as the skull and ribcage, also help to protect the
internal organs, such as the brain, heart and lungs, from damage. The skeletal
system and the
system also support the body and allow it to move away from danger,
toward food sources, etc.
Control and Regulation
To keep itself in a state of equilibrium, an organism must constantly gather information and
react accordingly. In humans, the
system, made up of the brain, nerves, spinal cord and sensory
organs, reacts to stimuli in the environment and signals other systems when
actions are needed to bring the body back into balance. The
system, which produces hormones and other regulatory substances,
plays a key role in maintaining balance among chemical messengers within the
body.
Several organ systems control these various vital functions throughout the body. Since
the organ systems control large regions of the human body, it is necessary to define
orientation within the body and communicate the proper terminology as you study these
integrated structures and functions.
To better identify the locations of the organs that contribute to vital
functions, you need some points of reference for description. To serve that
function, we will now define different planes of the body. These imaginary flat
surfaces run through the body in different directions. They are used by medical
professionals to examine various internal body parts. Directional orientation is
another anatomical tool used to describe how parts of the body are related to
one another.
Each organ system spans large regions of the human body. It is helpful,
therefore, to establish reference planes and directions that can help us
describe specific locations of structures as we discuss them. To make sure
everyone is talking about the same thing, anatomists and physiologists often
refer to anatomical position and the body planes that penetrate it. Anatomical
position describes a person standing upright, with the arms at the sides and the
palms facing forward (as demonstrated in the image below). Body planes (a plane
is a flat, two-dimensional surface) are imaginary surfaces that run through the
body and divide it into different sections. We can talk about a specific
location using the planes as reference points within the anatomical position.
There are an infinite number of planes running through the human body in all
directions. However, we will focus on the three planes that are traditionally
used when discussing human anatomy. First is the , (also called the horizontal plane), which divides the body into top
and bottom. In anatomical position, transverse planes are parallel to the
ground. The second is the , which is a vertical plane that divides the body into the front and
back sections. If you do a “belly flop” into the water, you sink into the water
via the coronal planes. Finally, we will refer to the , which divides the body into left and right sections with a vertical
plane that passes from the front to the rear.
This example shows planes passing through midpoint of body, producing two equal
sections. The plane, however, can be positioned at any point along the body's
horizontal or vertical axis, while maintaining the same direction of the plane;
this would produce unequal sections.
Example
Body Planes in Medicine
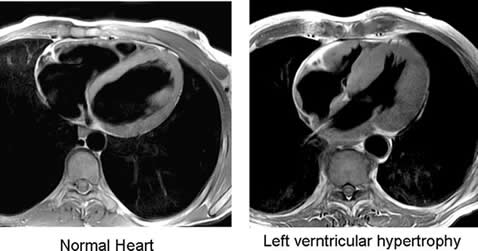
Many imaging modalities used in medicine (CT scans, MRI scans and
ultrasounds) image the body in cross sections. The three main planes
described above are used to orient and describe where the cross section is
and how it passes through the body so that the viewer knows what they are
viewing. For example, the image below from a CT (or CAT) scan shows a cross
section of the body that runs along the sagittal plane .
These images can sometimes be reconstructed in a computer to show the same
body in a different plane, making some features of the body easier to see.
Below is a coronal reconstruction of a CT:
You can use other terms to further pinpoint an anatomical location. These terms are used
to describe a location in relation to other structures. Some of them may be terms you
have heard in everyday conversation; a lateral pass in football, for example, is a pass
toward the sideline.
Superior, Inferior, Anterior and Posterior
The first set of directions that we will explore are , , , and .
In humans, which stand upright on two feet, there are other terms that are
synonymous with these four terms. Cephalic means toward the head and is the same
as superior for a human in anatomical position. Caudal means toward the tail, or
same as inferior for a human in anatomical position. Dorsal means toward the
back and ventral means toward the belly; so dorsal and posterior are the same
direction and ventral and anterior are the same direction for a human in
anatomical position. This would not be true for a four-legged animal, such as a
rat or cat you might dissect in lab.
Medial, Lateral, Intermediate
Next, we will discuss terms that
relate
structures to the
midline.
These are , and .
Proximal, Distal, Superficial, Deep
These next terms are used when referring to either appendicular parts of the body
(arms and legs) or position in body relative to the external surface. These are , , ,
The following table lists all of the human anatomical directions that we discussed. You
will practice using these planes and directional terms when describing the locations of
organs and organ systems in the following sections.
| Directional Term |
Meaning |
| (cephalic) |
above (or toward the head) |
| (caudal) |
below (or toward the feet or tail) |
| |
between |
| |
farther from the trunk |
| |
closer to the trunk |
| |
toward or on the surface |
| (internal) |
away from the surface |
| |
toward the front (or toward the belly) |
| |
toward the rear (or toward the back) |
| |
toward the
midline |
| |
toward the side |
did I get this
Directional Terms in Medicine
Now that you have reviewed ways to describe location and orientation, you will learn
about the organ systems that are necessary for the vital functions of life. You will
also get a chance to practice using body planes and directional orientations to explain
the anatomical integration and relative location of structures within organ systems. The
next section will systematically describe the organ systems of the body, as well as the
major anatomical structures and functions.
- Organ System
-
(Definition)
An organ system is an integrated collection of organs in the body that work
together to perform a vital function. This course will organize the organ
systems of the body based on the vital functions defined earlier.
The major organ systems of the body are shown in the table below.
| Major Organ Systems of the Body Grouped by Primary Function |
|---|
| Function |
Organ System |
| Exchange with the Environment |
System |
| System |
| System |
| Fluids and Transport within the Body |
System |
| System and Immunity
|
| Structure, Support, Protection and Movement |
|
| System |
| System |
| Control and Regulation |
System |
| System |
|
Example
Skeletal System
As an example of how the components of an organ system work together, let’s look at
the skeletal system. The most obvious components of this system are the bones, which
form a rigid framework for the body. The bones contribute to our ability to stand
upright and move around, but they can’t do it alone. and are also parts of the skeletal system. Ligaments connect the bones to each
other. Cartilage cushions the spaces between the bones, allowing for smooth
movement. And the bones couldn’t move at all without the skeletal muscles, and that connect muscles to bones (parts of the muscular system). The bones
provide the muscles with something to pull against.
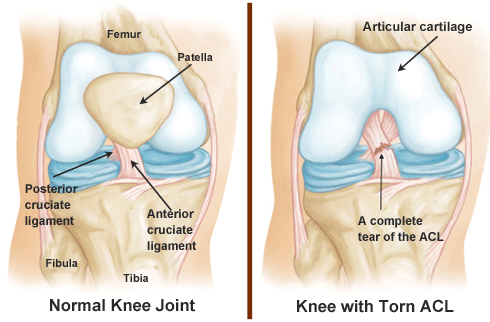
If one component of an organ system is damaged or malfunctions, the function of the
organ system will be affected. Think about a broken bone. If the femur breaks, it
will be much harder to maintain an upright posture, or to walk or run. These might
also be more difficult if the cartilage of the femur is destroyed by arthritis or a
ligament in the knee is injured while playing a sport. If any component of the
skeletal system is damaged—bone, ligament or cartilage—the knee will not function
properly.
The sections that follow will describe the details of the
organ systems that perform the
vital functions of life. You will learn how they contribute
to homeostasis and how imbalances in homeostasis lead to various disease
states.

Digestive System - Function
Also called the
system, the digestive system breaks down eaten material into
nutrient molecules, absorbs water and ions, and eliminates undigested residue.
The digestive system is a continuous tube (the digestive tract or alimentary
canal). Areas along this tube are specialized to perform different functions
related to getting the nutrients from your food to the cells that need them.
Accessory organs add secretions into different areas along the tube.
Your cells can’t use the pizza you had for lunch in pizza form. It needs to be
broken down into molecules that are small enough to be absorbed. As the pizza
travels along the digestive tract, each organ along the way breaks it down
further. Muscles in the walls of the digestive tract keep things moving along,
and glands in the tissues secrete digestive juices—mostly enzymes and acids—that
break up the larger substances in the pizza into smaller molecules. Food is
physically broken into smaller pieces in a process termed mechanical digestion.
These pieces are then chemically broken down into smaller units in a process
termed chemical digestion. Proteins are broken down into amino acids.
Carbohydrates are broken down into simple sugars. Fats are broken down into
molecules like fatty acids and cholesterol. It is important that the large
particles are broken into their smallest units so they can be absorbed from the
digestive tract into the bloodstream. Therefore, the main functions of the
digestive system are to ingest, break down, and absorb the nutrients from our
food. It also eliminates the wastes (anything not absorbed) as feces.
Digestive System - Organs
The specialized organs of the digestive tract extend in a roughly superior to
inferior direction from the mouth (where food goes in) to the anus (where waste
comes out) in the following order:
- Mouth
-
-
- Stomach
- Small intestine (including the duodenum, jejunum, and ileum)
- Large Intestine (including the cecum, colon, and )
- Anus
Accessory organs in the digestive system are connected to the digestive tract and
secrete additional digestive juices.
The produce saliva containing (among others) amylase, an enzyme that breaks
down carbohydrates.
The secretes a variety of enzymes that break down fats, carbohydrates, and
proteins, as well as bicarbonate ions that neutralize stomach acids. It is
important to note that this function corresponds to the exocrine portion of the
pancreas.
The liver produces bile, which aids in fat digestion and
absorption.
The gall bladder stores and concentrates bile and secretes it into
the small intestine.
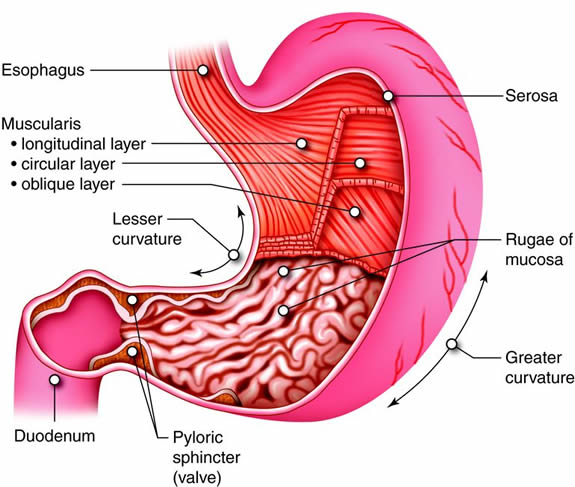
The stomach is a sort of muscular sac that can expand to hold a
large meal. Glands in the walls of the stomach secrete enzymes and acids that
break down food. Muscles in the walls of the stomach churn the food and
digestive juices together. Although the stomach can receive a large amount of
food at a time, it releases its contents gradually into the small intestine, so
that the intestine can better perform its function.
Digestion continues in the small intestine, with additional
digestive juices produced by the pancreas, liver, and the walls of the small
intestine itself. The walls of the small intestine have numerous tiny folds,
which increase its surface area, allowing for efficient absorption of nutrients
into the circulatory system, which in turn takes the nutrients to all the cells
of the body.
Excess water is reabsorbed in the large intestine, and the
undigested portion of your pizza leaves
the
body. Resident microbes of the large intestine (gut
microbiota) can digest substances that our cells cannot.
Digestive System - Anatomy and Direction
The digestive system is located primarily in the abdomen.

Respiratory System - Function
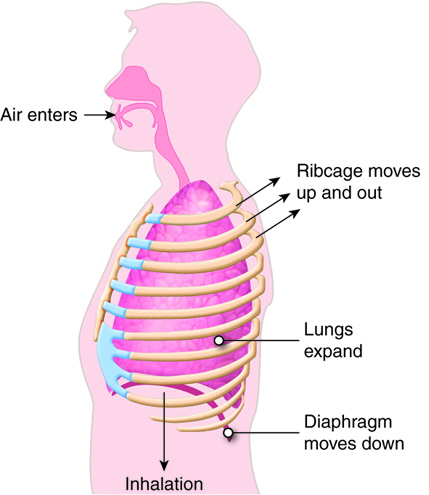
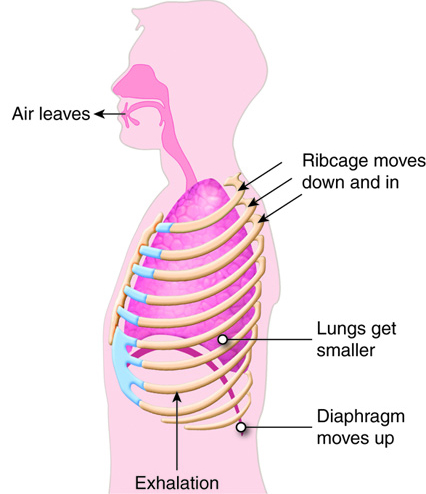
When humans breathe, air enters and exits via the respiratory system. This allows
the body to obtain oxygen, which is needed for metabolic processes, and
eliminate carbon dioxide, which is a metabolic waste product and can affect the
body's pH homeostasis.
Like the digestive system, the respiratory system can be thought of as a tube, or
rather, as a branching series of tubes that get smaller and smaller as they
branch off. Unlike the digestive system, which moves solids and liquids in a
single direction, the respiratory system moves gases in both directions, when we
inhale and exhale.
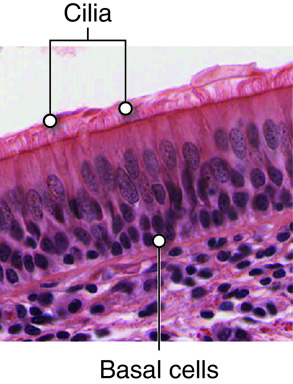
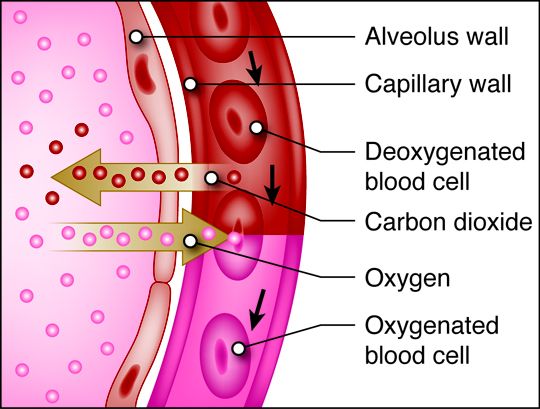
When we inhale, air passes through the nose or mouth into the pharynx,
, , lungs, and into smaller and smaller airways termed bronchi and then bronchioles,
until it reaches the air sacs, or . Only a single cell thick,
the walls of the alveoli allow the oxygen in air to diffuse into the blood, and
the cardiovascular system carries it to each cell in the body.

Carbon dioxide, a waste product of cell metabolism,
also diffuses through the alveolar walls, but in the opposite direction,
from the blood to the airways. Carbon dioxide is then exhaled through
the airways to the external environment.
Respiratory System - Organs
The organs of the respiratory system are arranged in a roughly superior to inferior direction and include:
- Nose
- Mouth
- Pharynx
- Larynx
- Trachea
- Lungs
Within the lungs, the respiratory system can be further divided into:
- (singular bronchus)
-
- Alveoli (singular alveolus)
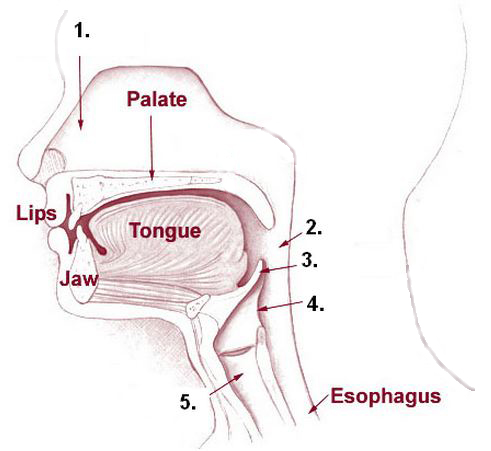
Note that the pharynx (the part of the throat just behind the mouth) is listed as a part of both the digestive and respiratory systems.
- Food and beverages pass through the pharynx on the way through the digestive tract.
- Air passes through the pharynx on its way to and from the lungs.
Food and water are prevented from entering the airway when we swallow by a structure
called the epiglottis. It is not uncommon for organs to be part of more than one
organ system. The pancreas, for example, has both digestive and endocrine
functions, and the kidneys play a role in both the urinary and endocrine
systems.
Respiratory System - Anatomy and Direction
The respiratory system is superior to the abdomen and internal to the ribs.

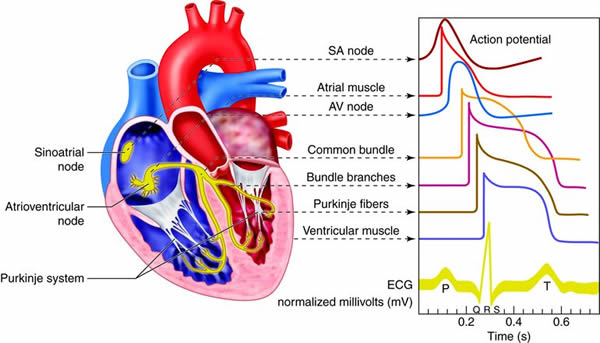
Cardiovascular System - Function
The cardiovascular system transports, from one part of the body to another:
nutrients, oxygen, ions, proteins, hormones and other signaling molecules, as
well as waste products, including carbon dioxide. This system also helps to
maintain homeostasis of fluid volume, pH, and temperature.
Cardiovascular System - Organs
The primary components of the cardiovascular system are blood, the heart, and the
vessels of the circulatory system, which work together to transport nutrients,
wastes, and gases to every cell in the body.
The blood that is circulated throughout the body contains two main
components:
-
Plasma contains water, electrolytes, glucose, proteins
(including enzymes, hormones, and blood clotting factors) and metabolic
wastes
- Formed elements or blood cells
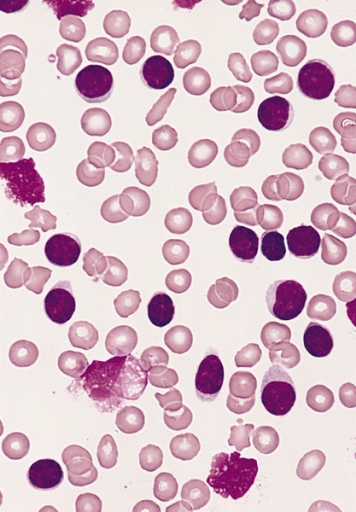
There are three types of formed elements:
-
Red blood cells transport oxygen and carbon dioxide.
-
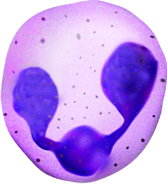
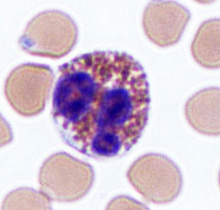
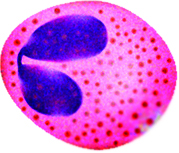
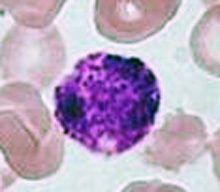
White blood cells fight infection by attacking foreign cells,
and clear old or diseased cells.
-
Platelets are important for hemostasis (not to be confused with
homeostasis); hemostasis is our ability to stop bleeding after vascular
injury (injury to blood vessels).
The blood functions to transport molecules and blood cells and contributes to the
maintenance of pH balance. Blood cells are formed in the red bone marrow.
The heart is divided into four chambers. The two lower chambers, called
ventricles, force blood out into the arteries. The two upper chambers of the
heart, called atria, receive blood returning from the veins. The heart contracts
as a unit, both atria (named right and left) contract together to move blood
into the ventricles and then both ventricles contract at the same time to move
blood out of the heart into the pulmonary artery and the aorta.

The cardiovascular system is divided into two functional subsystems.
- The systemic circuit transports blood and its components to
the body.
- The pulmonary circuit transports blood and its components
between the heart and the lungs.
Arteries of the systemic circuit (also
known as the
systemic circulatory circuit) carry oxygenated blood
from your heart to provide
oxygen and nutrients dissolved in the blood to every
cell in your body. When
blood leaves the left ventricle it first enters the
aorta, the largest artery in
the human body. Arteries gradually branch into
smaller and more numerous
arterioles which then supply blood to the smallest
vessels, termed capillaries.
It is estimated that your body contains
approximately 60,000 miles of
capillaries, that is enough to encircle earth three
times! Capillaries allow the
exchange of oxygen, nutrients and waste between the
blood and tissue cells. After waste has been picked up, blood
is moved through vessels of increasing size venules
into the larger veins. Veins return
oxygen-poor blood back to the heart, where the blood
is passed to the pulmonary
circuit to the lungs to pick up oxygen.

The pulmonary artery (part of the pulmonary circuit) carries
oxygen-poor blood from the right ventricle of the heart to the lungs for
oxygenation and removal of carbon dioxide. The pulmonary veins
carry oxygenated blood from the lungs to the left side of the heart.

Without this system in place that involves both the pumping of the heart to
squeeze blood out, and the network of vessels to distribute the pumped blood,
the cells of your body would not have an adequate supply of nutrients and
oxygen.
Cardiovascular System - Anatomy and Direction
The heart lies medial to the lungs, anterior to the spinal cord,
posterior to the sternum, and superior to the diaphragm. The heart is divided
into four chambers. The two lower chambers, called ventricles,
force blood out into the arteries. The two upper chambers of the heart, called
atria, receive blood returning from the veins.

Lymphatic System and Immunity - Function
Our body is in constant exchange with the environment, through breathing,
eating
and other activities. Therefore, it is important to screen the body and its
components regularly to identify foreign invaders that might enter during these
activities (or in any other manner). Further, it is important to rapidly and
effectively remove these invaders before they can cause significant harm. Our
body has specialized transport systems to carry out these functions. The
cardiovascular and lymphatic systems work together to transport excess fluids
(blood and lymph fluid, respectively) away from body tissues. Once fluid enters
the lymphatic system it is termed lymph. Lymph travels through lymph vessels and
passes through many lymph nodes which filter and clean the lymph. The immune
system also produces and matures immune cells, which protect the body from
invasion by agents that cause disease. One additional function of the lymphatic
system is to transport absorbed fat from the digestive system to the body
cells.
The immune system coordinates the activities required to respond to disease and
infection. This response can provide two types of immunity:
-
Specific immunity, in which specialized cells (such as T and B
cells) recognize specific foreign molecules called antigens within the body
and respond to them.
-
Nonspecific immunity, in which the body uses several general
methods such as physical barriers (that is, the skin and mucous membranes),
fever, inflammation, specific action by immune cells, and enzyme activity to
protect itself against general harmful agents.
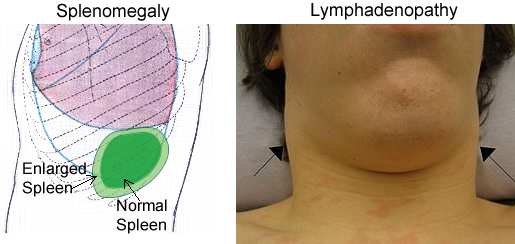
Lymphatic System and Immunity - Organs

The major organs of the lymphatic and immune systems (described below) can be
classified based on their role in lymphocyte (a type of white blood cell)
maturation. Maturation of lymphocytes takes place within the red bone marrow and
the thymus gland, which are primary lymphoid organs. Antigens become trapped
within secondary lymphoid organs such as the lymph nodes, spleen, and tonsils.
These organs are sites that contain lymphocytes for destruction of invading
pathogens.
| Organ |
Description |
| Tonsils and Adenoids |
Adenoids are one of three sets of tonsils. They trap pathogens that
enter through the mouth and nose. Also, the tonsils monitor the external
environment that the mouth and nose are exposed to, and can react with
an appropriate immune response for certain pathogens. |
| Thymus |
A lobular (of or pertaining to a lobe) structure, which contains many
immature, inactive lymphocytes. As the lymphocytes mature, they leave
the thymus to attack infected cells in lymphatic tissues throughout the
body. |
| Spleen |
The largest of the lymphatic organs, it houses lymphocytes for potential
immune response. Also, the resident phagocytes within the spleen perform
the most basic function of removing cell debris from the blood. |
| Lymph Nodes |
These house lymphocytes and macrophages, which destroy foreign material
contained in the lymph fluid. |
| Lymph Vessels |
These transport lymph fluid throughout the lymphatic system. |
| Red Bone Marrow |
All of our blood cells are generated from red bone marrow stem cells.
These stem cells differentiate into red blood cells, platelets, and
several cells that play roles in immunity. These “immune cells” include
lymphocytes, which carry out specific immunity, and neutrophils and
macrophages (macrophages start as monocytes and mature into macrophages
in the tissues), which are nonspecific phagocytic cells. |
While many people know that we are protected from foreign micro-organisms by an
immune system, few people realize how the immune system is able to patrol the
entire body. White blood cells of the immune system are produced in the red bone
marrow and travel through the blood. They can leave blood capillaries to travel
through tissues. White blood cells are then able to remove dead or damaged cells
and "foreign" organisms they encounter and recognize specific foreign organisms
again if necessary. Additionally, lymph collects from tissues and circulates
through lymph vessels, making "rest stops" in discrete points throughout the
body called lymph nodes or lymph organs. In these nodes and organs, including
the spleen, tonsils and other tissue clusters, there are large collections of
white blood immune cells. Lymph slowly travels through these organs ensuring
that the lymphocytes have plenty of time to react to these foreign organisms in
the lymph before returning it to the blood.
Lymphatic System and Immunity - Anatomy and Direction
The lymph nodes are located in several regions along the path of lymphatic
vessels in our body. The thymus is located within the upper chest, lies
posterior to the upper portion of the sternum, and extends from the root of the
neck onto the pericardium. The spleen is found in the upper left abdominal
cavity; it lies superior, posterior, and lateral to the stomach. The tonsils are
masses of lymphatic tissue within the nasopharyngeal (nose and mouth) region.

Urinary System - Function
The urinary system filters blood and adjusts the composition of
blood/interstitial fluid by removing excess water, salt, acid, and metabolic
waste from the body as urine. This allows the urinary system to control body
fluid volume, blood pressure, pH, and electrolyte balance. It is a critical
system for maintaining homeostasis.
We have seen how the digestive and respiratory systems remove some wastes from
the body—undigested food leaves the digestive tract through the anus and carbon
dioxide leaves through the lungs and airways. The urinary system (or excretory
system) filters blood to remove excess water, electrolytes and other metabolic
wastes and reabsorbs water, electrolytes and other molecules as needed to
maintain homeostasis in the body fluid. The resulting excess and wastes are
excreted as urine. In this way, the urinary system also works with the
respiratory system to maintain pH balance in the body.
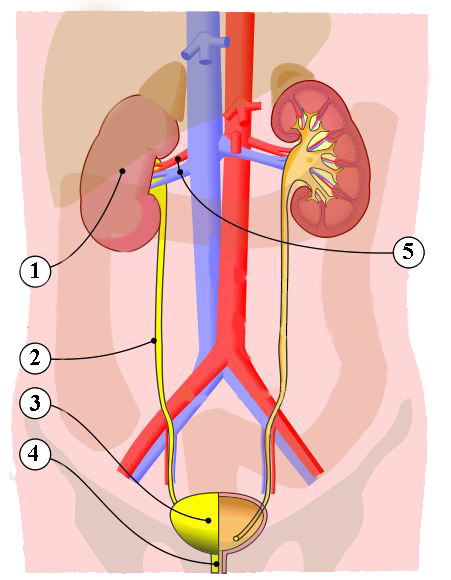
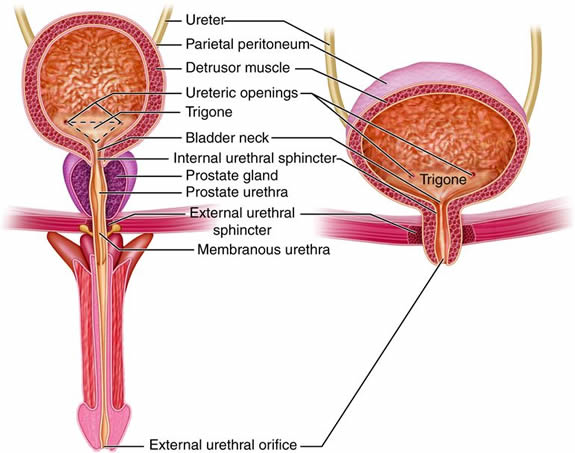
Urinary System - Organs
The organs of the urinary system include:
- Kidneys
- Ureters
- Urinary Bladder
- Urethra
The kidneys, the main organs of the urinary system, are located
against the posterior wall of the abdomen. They serve as a filtration and
reabsorption system, where soluble substances are filtered and then those that
the body needs to keep are reabsorbed. Those that are not reabsorbed (or not
reabsorbed fully), such as our metabolic waste products, end up in the urine.
Because our bodies are constantly producing wastes, the kidneys continuously
work to prevent the buildup of waste products and toxins, filtering about 180
liters of fluid a day. Because the average person has about three liters of
plasma (the fluid fraction of blood), this means that our plasma is filtered
about 60 times a day!
One of the most important of the waste products removed from the blood is urea,
the main end product of protein metabolism. Other waste products and some toxins
are also removed from the blood by the kidneys.
In addition, ions and water are also filtered by the kidneys, but a large
fraction of these are reabsorbed to keep the fluid and electrolyte concentration
of the blood and other body fluids within an optimal range for proper cell
function. The kidneys also play an important role in the regulation of pH by
managing the amount of acid in the urine.
As previously described the kidneys filter the blood to form urine. Urine leaves the kidneys and flows through the to the urinary bladder, where it is stored until it passes out of the
body through the . On average, two liters of urine are produced per day, but this can
vary greatly depending upon fluid intake, fluid loss through perspiration, and
other factors.
Urinary System - Anatomy and Direction
The right and left kidneys are located against the posterior wall of the
abdominal cavity. The kidneys’ location is also described as retroperitoneal
because they are behind the peritoneal cavity that encloses the intestines.

Integumentary System - Function
We often don't think of the skin as a complex organ, but it is. The skin is the primary organ in the
integumentary system, which also includes hair, nails, and certain glands. The integumentary system
helps to provide support and structure for the body, but it also plays several other important roles:
- It is the first line of defense against foreign organisms and the external environment.
- It helps to regulate body temperature.
- It senses changes in the environment (pain, pressure, touch).
- It supports the removal of wastes (as sweat).
- It aids in the production of vitamin D.
The integumentary system is one of the most active parts of our body, even though
we are not as aware of its activity as we are with the heart, lungs or stomach.
The integumentary system encapsulates and protects the body. The skin is
actually the largest organ in the body because of its large surface area. In
some ways, the skin can be thought of as an immune system organ, since it
protects the body from foreign organisms. In other ways the skin can be thought
of as a sensory organ because it contains many nerves that are related to the
sense of touch. The skin also integrates with muscles and allows for movements
such as facial expression.
Integumentary System - Organs and Structures

If we take a closer look at the skin, you can see that there
are many layers. Within the skin there are hair follicles from which
hair grows. Also, there are sweat glands that produce sweat for thermal
regulation of the body and sebaceous glands that secrete oil to
waterproof and moisturize the skin. Nails are also included in the
integumentary system, as are horns, feathers, claws and hooves...but
hopefully you don't have any of those.
The major structures within the integumentary system are:
- skin
- hair, nails
- sebaceous glands
- sweat glands
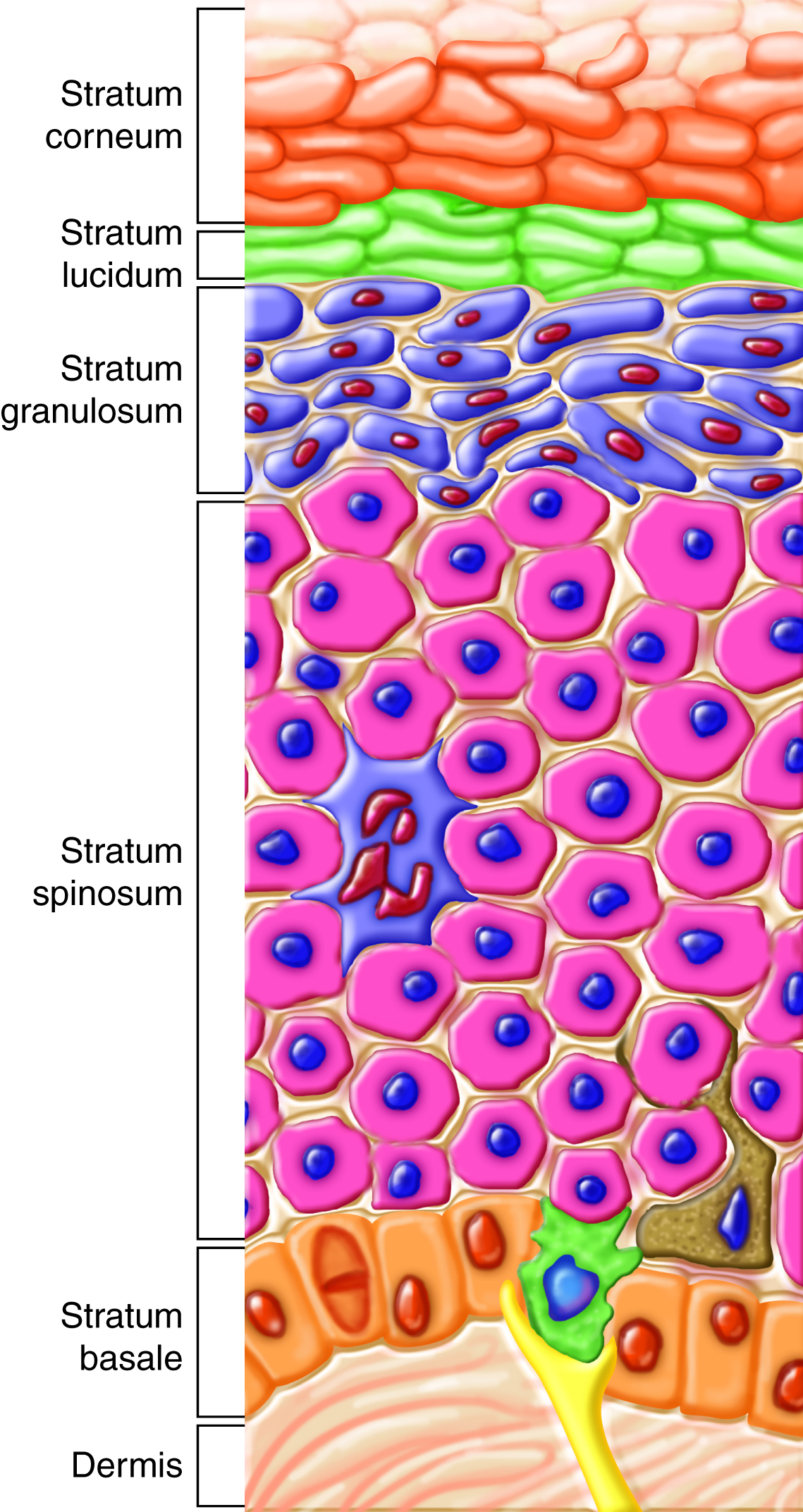
Skin, the largest organ of the body, is the primary organ of the integumentary
system. Skin is composed of three main layers, each of which has specific
functions related to its structure. The three main layers of the skin are:
-
epidermis, which acts as a seamless, waterproof barrier to the
external environment and prevents excessive water loss from the body (the
root "epi-" means "above").
-
dermis, which provides the tensile strength and elasticity of the skin, contains nerves and sensory receptors and
contains blood vessels that aid in regulating body temperature.
-
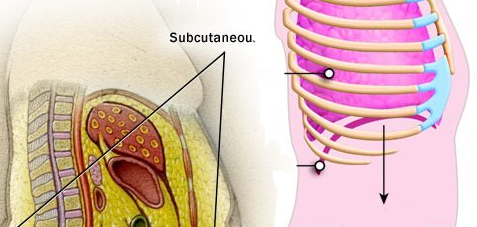
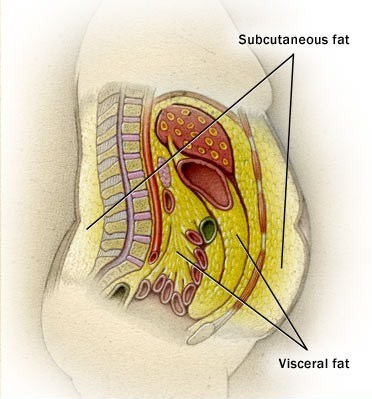
hypodermis, which attaches skin to other structures below it and acts
as an insulator and shock absorber (the root "hypo-" means "below"); the
hypodermis is also known as the subcutaneous layer.
Hair, another component of the integumentary system, is found in nearly all
regions of the skin, except on the palms, soles of the feet, and some parts of
the genitals. Hair grows from hair follicles that are part of the
epidermis, even though they extend down and the dermis extends up around them.
Hair helps regulate body temperature and protect the surface of the body,
including eyelashes that protect the eyes.
Nails are located on the end of each distal phalanx (each finger and each toe).
They protect the phalanges from trauma, and provide mechanical support for
manipulating objects. Nails grow from epidermal cells in the nail beds.
Glandular structures are also part of the epidermis, and are present in different regions
of the skin. They secrete substances that are important for many physiological
functions. There are three main types of glands:
-
sebaceous glands, whose secretions maintain the softness and hydrophobicity
(water repellency) of the hair and skin.
-
apocrine glands, whose secretions moisten the skin during pain, fear, sexual arousal and emotional upset.
-
merocrine (eccrine) glands, which secrete sweat to regulate body temperature.
Integumentary System - Anatomy and Direction
The skin covers the outside surface of the body. Special structures such as hair, nails and glands are
part of the integumentary system.

|

|
Skeletal System - Function
The skeletal system, which includes the skeleton and articulations (joints),
provides support and protection for soft tissues and organs, aids in movement,
serves as a reservoir of calcium, and produces all blood cells.
Although we often think of bones as the only organs of the skeletal system,
cartilage, ligaments and tendons are equally important organs. These structures
of the skeletal system work together to:
- provide leverage and resist forces from muscles and gravity.
- keep joints together.
- allow flexibility within joints.
- maintain a safe range of motion.
Bones are found throughout the body from the skull in the head to the 26 bones
in the foot. Bones allow us to maintain our stature, they protect softer
internal organs, and they let us move around. Bones are interconnected by
articulations, another word for joints. In an articulation, where bone meets
bone, there is a layer of softer cartilage. Articulations are then stabilized by
ligaments, which help keep the bones aligned properly. Bones are connected to
the muscular system by tendons, which allow the body to move.
Skeletal System - Organs

The major structures within the skeletal system are:
- bones
- cartilage
- ligaments
- tendons
The skeletal system consists of bones, ligaments, tendons, and cartilage. Bone is
the primary organ of the skeletal system. Although there are different types of
bones in our body, the basic components of all bone tissue are the same:
- Osteoblasts, osteoclasts, and osteocytes, which are specialized cells that
are responsible for bone formation, regulation and repair.
- Collagens and other proteins, which give bone its flexibility.
- Inorganic calcium and phosphate minerals, which give bone its hardness.
- Red bone marrow, which produces all blood cells in a process termed hematopoiesis (or hemopoiesis).
These basic components give bone tissue its load-bearing, protective qualities.
The living cells in bone allow it to sense and respond to stress. The inorganic
matrix of bone gives the bone rigidity and also acts as a storage depot for
calcium and phosphorus in the body.
Cartilage is a firm, flexible, and smooth connective tissue found at
the ends of bones. Cartilage is present in joints to protect the bone and to
evenly distribute forces to the underlying bone.
Ligaments are band-like elastic structures that surround joints to
hold them together. Ligaments connect one bone to another bone, and allow
movement in very specific directions.
Tendons are band-like structures similar to ligaments. However,
tendons are more rigid and connect bones to muscles. Tendons play a role in
integrating the force generation of the muscle with the rigid bone, which helps
actuate large-scale motion.
The numerous organs and structures of the skeletal
system allow it to serve an
important role in the support and protection of our
body. Bones are very strong,
yet flexible which makes them perfect for supporting
our weight and allowing
movement. The connective tissues such as cartilage,
ligaments, and tendons aid
in protecting our joints and providing stability.
The red bone marrow inside the
bone is vital for hematopoiesis or the production of
all blood cells. Bones are
also a reservoir for calcium. If your diet is
deficient in calcium, a hormone will mobilize calcium from the bones to
the blood, and your bones will be weaker.
Skeletal System - Anatomy and Direction
Bones are found throughout the body. Regions capable of more intricate movements,
such as the hands and feet, have more articulations and therefore more bones.
Each articulation has cartilage and is stabilized by ligaments.

|

|
Muscular System - Function
The muscular (musculoskeletal) system generates force for movement of bones
around articulations, facial expression, breathing, posture, and assists with
temperature regulation. The muscular system only contains skeletal muscle,
although the body also has smooth and cardiac muscle tissue, which are important
in other body systems. There are over 650 skeletal muscles in the human
body!
The skeletal muscle converts signals from the nervous system into movement via
muscle contractions. Muscles, like the biceps and triceps, are the organs of the
skeletal muscular system. The main functions of skeletal muscles include:
- responding to neural information (conscious control)
- applying forces to the bones to cause movement
- producing heat to warm the body
- changing the size of the thoracic cavity for breathing
- applying forces for conscious control of openings to the outside of the body
(sphincters)
The muscular system contains muscle tissues and interconnects with both the
nervous system and skeletal system. Nerves control the muscles and allow us to
consciously direct movements. Some muscles, such as the muscles that control the
pupil of your eye, cannot be controlled consciously but react to nerve stimuli.
The skeletal system provides a stiff support for muscles to pull on. Muscles
generate force to lift as well as to balance us. The energy produced by
contracting muscles (such as when shivering) in the muscle system helps keep us
warm. There are many muscle fiber types throughout the body that vary based on
function. Parallel muscles form along the long bones, pennate and convergent
muscle fibers attach to tendons and circular muscles assist with closing our
eyes or puckering our lips.
Muscular System - Organs
The major structures within the muscular system are:
Skeletal muscles are voluntary muscles that attach to, and contract
to move the bones. Skeletal muscles often work in pairs. When one muscle is
contracting, the other is relaxing. For example, to bend your arm at the elbow,
your biceps muscle contracts, and your triceps muscle relaxes. To straighten
your arm, the biceps relaxes, and the triceps contracts. The diaphragm is
skeletal muscle that contracts and relaxes for inhalation and exhalation.
Hiccups are a spasm in your diaphragm muscle.
Skeletal muscles are made of long cylinder shaped cells called muscle
fibers, which have many nuclei within each cell. Therefore we say
that skeletal muscle is multi-nucleate. The functional unit within a skeletal
muscle fiber, called a sarcomere (note that “sarc” means flesh),
contains filaments of the proteins actin and myosin. Myosin is a thicker protein
(appears darker) than actin and the two proteins create a pattern so the muscle
appears striped or striated. Notice the appearance of skeletal muscle in this
transmission electron microscope view.
 Sarcomere - By Louisa Howard Human skeletal muscle tissue 1). Public Domain.
Sarcomere - By Louisa Howard Human skeletal muscle tissue 1). Public Domain.
A muscle contraction occurs when the myosin filaments pull on the actin to
shorten the sarcomere. This results in shortening of the muscle fiber and
ultimately the entire muscle shortens or contracts to pull on the bone.
An electrical signal from the nervous system is necessary to cause a skeletal
muscle contraction. The area where the nerve meets the muscle to stimulate it is
termed neuromuscular junction. When a nerve signal reaches the
neuromuscular junction, the muscle fiber is stimulated and the muscle contracts.
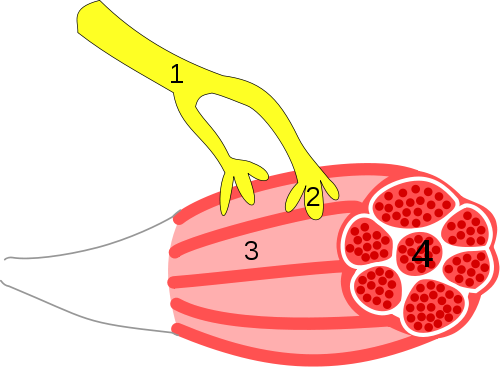
In the image below the #1 is termed the axon or the part of a neuron that carries
the instructions from the brain and spinal cord. #2 is the end of the axon
called the axon terminal or synaptic vesicle. #3 is the muscle and #4 is a group
or bundle of muscle fibers.
 Neuromuscular Junction - By Synapse_diag3.png: User:
DakeMusculus_diagram.svg: *Skeletal_muscle.jpg: User:Deglr6328derivative
work: Marek M (talk)derivative work: Marek M
Neuromuscular.svg)CC-BY-SA-3.0
Neuromuscular Junction - By Synapse_diag3.png: User:
DakeMusculus_diagram.svg: *Skeletal_muscle.jpg: User:Deglr6328derivative
work: Marek M (talk)derivative work: Marek M
Neuromuscular.svg)CC-BY-SA-3.0
are grouped in both the skeletal system and the muscular system since
they connect the two systems (connect muscle to bone). Tendons play a role in
transmitting force from the muscles to the bones to permit movement.
Other Muscle Types
Although only skeletal muscle is part of the muscular system, there are three
types of muscle tissue. Smooth muscle and cardiac muscle are similar to skeletal
muscle, but perform specialized functions in the body. Most of these functions
are involuntary and do not include the skeletal system.
Smooth muscles control involuntary functions of the body, such as
arterial contractions to move blood and peristaltic contractions in the
digestive system to move food. Smooth muscles lack striations thus, are termed
smooth due to their appearance. They are composed of muscle fibers with a single
nucleus in each cell and are uninucleate. Smooth muscles do not have any
attachment to the skeletal system. Smooth muscle has the ability to produce its
own contractions involuntarily. However, as with skeletal muscle, electrical
signals from the nervous system can modulate the activities of smooth muscle.
The organization of smooth muscle on a cellular level is irregular and
unorganized. Therefore, smooth muscle does not contain sarcomeres.
Cardiac muscle contains similarities to both skeletal and smooth
muscle. Like skeletal muscle, cardiac muscle is composed of organized muscle
fibers and sarcomeres, and is striated. However, cardiac muscle does not attach
to the skeletal system and is under involuntary control, and is uninucleate.
Cardiac muscle is not long and cylinder shaped like skeletal muscle but is more
branched.
Muscular System - Anatomy and Direction
Like bones, skeletal muscles are found throughout the body. Skeletal muscles are
found under the skin of the integumentary system and attached to and surrounding
the bones of the skeletal system.

Nervous System - Function
The basic functional units of the nervous system that transmit messages are cells
called neurons. Signals travel through a neuron as electrical
impulses. Neurons release chemical substances, known as
neurotransmitters, to transmit information to other neurons, to
muscles, or to glands. The chemical messages of the nervous system are
transmitted over short distances, and their effects are short-lived. The nervous
system allows for control and coordination of skeletal muscular movements that
may be consciously predetermined, or may happen automatically, such as reflexes.
Other parts of the nervous system control and coordinate subconscious body
activities, including heart rate, gland secretions and smooth muscle movement in
the digestive system. Some activities, such as breathing, can be controlled both
subconsciously and consciously. The nervous system typically works quickly. It
also allows us to integrate and store information, such as when you are
learning.
The nervous system transmits signals to different parts of the body to
coordinate function. Electrochemical signals are processed in the brain and sent
down the spinal cord, which runs the length of the back. From the spinal cord,
peripheral nerves send signals out to the extremities. Return signals come in
through sensory nerves and either return to the spinal cord for processing or
back to the brain. The spinal cord processes reflexes and repeated patterns.
Nervous System - Organs and Structures
The nervous system is often divided into two functional parts:
- The central nervous system, which processes incoming
information and initiates a response.
- The peripheral nervous system, which brings sensory information
to, or carries motor output from, the central nervous system to initiate a
reaction.

Central Nervous System
The major structures within the central nervous system are:

The brain has several lobes, each of which carries out
specific functions and processes information associated with specific
parts of the body. The spinal cord is located within the
vertebral column and processes some reflexes but primarily transmits
information to and from the brain along neurons. Specialized membranes
called meninges cover the brain and the spinal cord to
protect them. Additionally, a special fluid, called cerebrospinal
fluid, chemically and mechanically protects the brain and
spinal cord.
Peripheral Nervous System
The major structures within the peripheral nervous system are:
- cranial nerves
- spinal nerves
The peripheral nervous system is composed of nerves outside the brain and
spinal cord. Nerves are bundles of extensions from neurons that extend
through the body in the peripheral nervous system. These nerves are
categorized into the following functional groups:
-
sensory nerves, which carry sensory input to the brain
or spinal cord from the environment.
-
motor nerves, which carry motor impulses from the brain
or spinal cord to muscles or glands.
-
mixed nerves, which have a combination of sensory and
motor neurons in one nerve.
The peripheral nervous system can be subdivided into two subdivisions:
the somatic and autonomic divisions. The somatic nervous system includes
sensory neurons that send sensory information from sensory receptors of
the skeletal muscle, skin and special senses (including smell, taste,
sight, hearing and equilibrium) to the central nervous system and motor
neurons that control skeletal muscle.
The autonomic nervous system monitors and regulates changes
in the body's internal environment. These changes are not under
voluntary control. Body processes controlled by the autonomic nervous
system include the contractions of the stomach and other digestive
organs, the heart rate, and contractions of blood vessels to control
blood pressure and flow though the body.
The autonomic nervous system is further divided into the sympathetic and
parasympathetic divisions. The sympathetic nervous system
controls functions that speed up the heart and increase energy usage
during emergencies or times of stress. On the other hand, the
parasympathetic nervous system controls functions that
have the opposite effect—they reduce heart rate and decrease overall
energy usage when the body is returning to normal after an emergency or
during normal functioning.

Nervous System - Anatomy and Direction
The brain is protected inside the skull. The spinal cord runs from the
brain down through the bones of the spinal column. From the brain and
spinal cord, nerves run throughout the body, including to the limbs.

Endocrine System - Function
The endocrine system is an equally important method of sending messages within
the body for control and coordination of multiple body systems. The functional
unit of the endocrine system is a gland, or a group of cells that secrete
chemicals called hormones. Hormones circulate throughout the body within the
bloodstream and act as long-term messengers. In comparison with
neurotransmitters, hormones act over long distances for a longer time.
Endocrine System - Organs and Structures
The major organs of the endocrine system are:
- hypothalamus
- pituitary gland
- pineal gland
- thyroid and parathyroid glands
- thymus (note that this gland is also part of the lymphatic system)
- adrenal glands
- pancreas (note that this gland is also part of the digestive system)
- gonads (that is, ovaries or testes—note that these glands are also part of
the reproductive system)

As a specialized part of the brain, the hypothalamus is an endocrine
gland that produces hormones that regulate many basic functions such as hunger,
thirst and sleep through control of the pituitary gland. The hypothalamus
receives sensory input from receptors and perceptual information from the brain,
such as changes in emotional state, temperature, and lighting.
The pituitary gland is sometimes called the master gland, because it
controls the release of hormones from many other endocrine glands.
The pineal gland secretes the hormone melatonin, which is important for
transmitting information about environmental lighting and inducing sleep.
The thyroid glands and parathyroid gland are located together in the
neck. The thyroid glands secrete hormones that regulate metabolism and calcium
levels. The parathyroid gland also secretes hormones that regulate calcium
levels.
The thymus gland secrets the hormones thymosin and thymopoietin that
stimulate the production of special lymphocytes (white blood cells) called
T-cells,
which play an important role in the immune system by attacking foreign or
abnormal cells.
The adrenal glands produce steroid hormones that regulate metabolic
functions during stress, kidney function, and sexual function. The adrenal
glands also secrete epinephrine (adrenaline) when stimulated by the autonomic
nervous system.
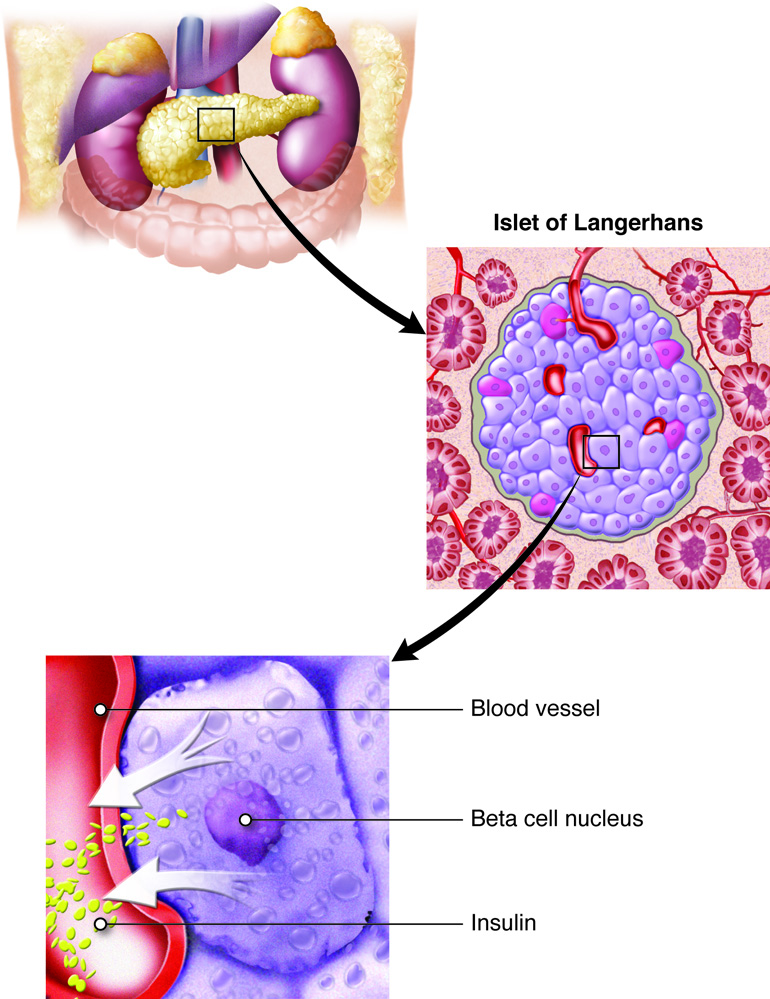
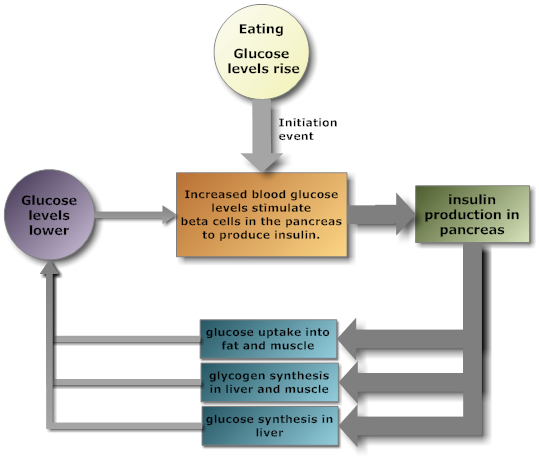
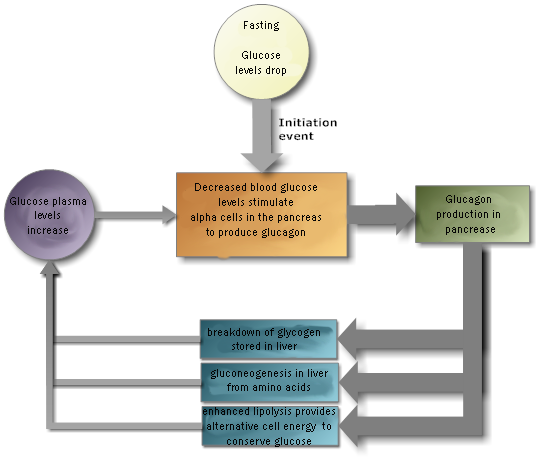
The pancreas secretes insulin, to lower blood sugar levels, and glucagon
to raise blood sugar levels. Therefore, the pancreas is an important endocrine
organ for regulating the fuels available for energy production by cells.
The gonads, or sex organs (ovaries and testes) secrete sex hormones
which control production of sperm and eggs as well as other secondary sex
characteristics that are different for males and females. The secretion of sex
hormones by the gonads is under the control of pituitary gland hormones.
Endocrine System - Anatomy and Direction
The hypothalamus is found deep inside the brain and lies inferior to the
thalamus. The pituitary gland is located at the base of the brain, inferior to
the hypothalamus. The pineal gland is a small gland on the midline at the
posterior of the brain. The thyroid and parathyroid glands lie inferior to the
larynx, around the trachea. The parathyroid gland lies on the posterior surface
of the thyroid gland. The adrenal glands are located superior to each kidney.
The pancreas is located posterior to the stomach and is connected to the part of
the small intestine called the duodenum. The ovaries are located in the pelvic
cavity lateral to the uterus, while the testes are held external to the
abdominopelvic cavity inside the scrotum.

Life is a complex continuum of flows of energy and matter. Discrete structures such as
organs and cells allow us to divide life into levels of organization. This organization
is to some extent artificial, and to some extent
practical.
The human body is a complex, hierarchical system—that is, a system made up
of smaller subsystems, which are themselves made up of even smaller systems. We commonly
study these different hierarchical levels—levels of organization—separately. By breaking
down the complex system into simpler parts, we can make the whole system easier to
understand. This “reductionist” approach, reducing a complex system to simpler
components, is central to how we practice modern science.
Regarding the body, therefore, we consider the body as a whole, then its subsystems, and
then the components of these subsystems. We can model the hierarchy of organization
within the body as comprised of organs, tissues, cells, cell organelles, macromolecules,
molecules and finally atoms.
The levels of organization that we will consider in this course are, from smallest to
largest:
- The chemical level, which consists of atoms, ions, and small
molecules
- The macromolecule level, which consists of large molecules
- The cell level, which consists of individual cells; this is the
smallest level that contains living entities
- The tissue level, which consists of groups of related cells working
together to perform a specific function
- The organ level, which consists of groups of tissues working together
to perform a higher-level function
- The organ system level, which consists of all of the organs involved in
performing a vital function
- The organism or whole body level, which consists of a
whole person
- The population or environment level, which involves the
interactions of the person with his or her environment
Although we will consider each level individually, it is important for you to keep in
mind the connections between the levels. Processes and events at one level can affect
other levels. An alteration in the structure of a protein (macromolecule level) can
prevent a cell from functioning properly; this improper function can affect the tissues,
organs, organ systems, and the whole body. And the reverse is true: changes to the body
(organism level) can affect organs, tissues, cells and molecules.
For example, suppose a single nitrogenous base in DNA (chemical level) is incorrect. This
mutation causes an alteration in the structure of the beta globin (β-globin) protein
(macromolecule level), which is part of hemoglobin. The altered structure of β-globin causes the proteins to stick
together and form fiber-like structures. Under certain physiological conditions, the
fibers in turn distort the shape of red blood cells (cell level), so that the cells
become curved and twisted. The abnormal cells get stuck in capillaries, reducing blood
flow to tissues and organs (tissue and organ levels). Organ damage may result,
permanently affecting body function (whole body level).
This example describes sickle cell anemia, a genetic blood disorder. We can clearly see
the connections between levels of organization. A seemingly tiny error at the genetic
(chemical) level causes significant changes in the body’s systems at higher levels. Many
genetic diseases arise in this way—through small alterations in the genetic code.
But scientists are homing in on the genetic basis for some diseases, such as cancer. In
some instances, our understanding may hint at genetic therapies for a disease. Such
technology is still largely experimental, but it shows the practical value of looking at
the levels of organization of complex systems.
At every level of organization, structure is related to function. For example water is
able to peform many of its unique and life-sustaining properties because of its
structure. It is a bent polar molecule that can form attractions with neighboring water
molecules through hydrogen bonds. At the macromolecular level, the unique structures
of enzymes allow these proteins to help speed up reactions. On the cell and tissue
level, the rigid matrix structure of your bones allows them to be able to support the
weight of your body. At the organ level, the "J" shape of the stomach allows for partial
segregation of its contents at the early stages of digestion.
Look around you. Everything is made of chemicals of one sort or another. Life is
chemistry organized into astonishing complexity and intricacy. To make sense of this
organization we can look at life’s chemistry as a hierarchy—levels of organization. From
simple elemental ions, to simple organic molecules, complexity rises with increasingly
larger macromolecules.
A person is between 1-2 meters (m) tall, but there are many length scales and biological
levels of detail which are important for understanding anatomy and physiology. For
perspective on size difference, considered an at 10-10 m. We can’t really grasp how small that is, but think big
instead of small. A length of 1010 m is more than the distance from the Earth
to the moon.
The smallest length scale that we will cover is the size of individual atoms, but the
movement of subatomic particles called electrons, can change atomic charge. Ions are
atoms that carry either a positive or negative charge from altered numbers of electrons,
and many atoms and molecules exist in the body as ions.
Ionic chemistry is important in human medicine and health. Ions play an essential role in
physiological processes, particularly in cell membranes. Sodium, potassium and calcium
ions are required for nerve impulses and heart beats, enable cell-to-cell communication
and initiate cellular processes. For example, release of insulin by beta cells of the
pancreas is mediated by ions. Transport of ions across membranes may occur by passive
diffusion, through ion channels, or through pumps. Pumps often move ions against a
concentration gradient. Ionic chemistry is important in human medicine. Anesthetic drugs
such as Novocain block sodium channels. Neurotoxins from some snakes and puffer fish
work by blocking ion movements in nerve transmission. Malfunctions in ionic channel or
pump molecules can result in serious physiological ailments, including cystic fibrosis
(mutation in a gene that codes for cell membrane chloride channel) and epilepsy.
Even subatomic particles, which are too small to see with the best microscopes in the
world, play an extremely important role in maintaining proper physiology.
The key biologically relevant elements are hydrogen (H), carbon (C),
nitrogen (N), oxygen (O), phosphorous
(P)
and sulfur (S). These elements represent more than 95 percent of the
mass of a cell. Carbon is a major component of nearly all biological molecules.
Elements are characterized by their atomic structure. While the subatomic structure of the
atom is a major topic of interest in chemistry, physics and biophysics, we will
only discuss the basic structure that will provide sufficient information for
the construction of molecules in the context of this course. Atoms have a
central nucleus with positively charged protons and neutral neutrons; negatively
charged electrons circle the nucleus. The electrons that are involved in
chemical bonding are those electrons in the outermost orbit, referred to as
valence electrons. On the periodic table below, you can view each
of the atoms while hiding all but the outermost electrons.
Atomic mass, the sum of the number of protons and neutrons in the atomic structure, is a
particularly useful measure of each element. By summing the atomic mass of all
the atoms in a molecule, one can estimate the molecular mass of the molecule,
which is then expressed in atomic mass
units,
or Daltons. This table
shows the masses of the six atoms of the elements listed above, which can also
be found in the upper right-hand corner of the box for each element in the
periodic table.
To calculate the mass of a
molecule,
we find the mass of each individual atom in the molecule and add them together.
For example, a water molecule (H2O) contains one oxygen atom that has
a mass of 16 amu (atomic mass units) and two hydrogen atoms that each have a
mass of one amu. Therefore, the mass of a water molecule is 16 amu + 2 X 1 amu =
18 amu.
The electronegativity of an element is the degree to which an atom will attract
electrons in a chemical bond. Elements with higher electronegativities, such as
N, O and F (fluorine), have a strong attraction for electrons in a chemical bond
and will therefore “pull” electrons away from less electronegative atoms.
Elements with low electronegativity, such as metals, tend to “give away”
electrons easily.
Some atoms with important biological relevance are shown. Place the mouse pointer over an element to
see its full name.
Chemical bonds result when atoms of the same element (e.g., C-C) or different
elements (e.g., C-O, C-N, O-H) are attracted to one another. There are two major
types of chemical bonds: ionic and covalent. A polar covalent bond is a type of
covalent bond that results in unique interaction between molecules. Some that contain polar bonds exhibit an intermolecular attraction or force
known as a hydrogen bond. Both chemical bonds and intermolecular forces are
important in the functioning of the cell.
Ionic Bonds
form when an atom or group of atoms gains or loses one or more
electrons. When an atom gains electrons, it becomes a negatively charged ion,
called an anion. When an atom loses electrons, it becomes a
positively charged ion called a cation. Atoms with higher
electronegativities tend to gain electrons and become anions, whereas those with
lower electronegativities tend to lose electrons and become cations. The
electrostatic attraction between a positively charged ion and a negatively
charged ion is the basis of an ionic bond.
An ionic bond generally forms between an atom of low electronegativity and an
atom of high electronegativity. In many cases this will be between a metal and a
nonmetal. In this situation, one or more electrons is transferred from the atom
with low electronegativity, which readily “gives away” its
electrons,
to the atom with high electronegativity, which strongly attracts those
electrons.
Example
For example, as illustrated in the animation below, a sodium atom will
transfer its one valence electron to a chlorine atom, resulting in the
formation of a sodium cation, Na+, and a chloride anion,
Cl-. Because these are oppositely charged particles, they are
attracted to each other and form table salt.
When an ionic compound, like table salt, is put into water, it dissolves. This
happens because the polar water molecule pulls these oppositely charged ions
apart, as will be discussed further in the next module.
Covalent Bonds
After single elemental atoms, we can think of small molecules as the next level
in chemistry’s hierarchy. Molecules result from the covalent bonding of two or
more elements’ atoms.
Covalent bonds are strong bonds in which electrons circling the atomic nucleus
are shared. The nature of the covalent bond is determined by the number of
electrons shared and the nature of the two elements sharing the bond. Two or
more atoms held together by covalent bonds in a specified arrangement is called
a molecule. The diagram below illustrates the covalent bond that forms between
two hydrogen atoms to form a molecule of hydrogen. Covalent bonds form between
atoms of the same or similar electronegativities, most often two nonmetals.
Each atom will most often form a specific number of covalent bonds when in a
molecule with other atoms. The number of bonds that a particular atom will form
is based on the atom’s valence electrons. Carbon for instance, which has four
valence electrons, will form four bonds when it is in a molecule, as you can see
from the diagram of methane below. Nitrogen, which has five valence electrons,
will form three bonds, as seen in the ammonia molecule. The number of covalent
bonds that a nonmetal will most commonly form is given in this table for the
biologically important elements.
The number of bonds between two atoms, such as single bonds, double bonds or
triple bonds, helps determine the stability of the atomic interactions. Double
bonds, which share two pairs of valence electrons between two atoms, are very
strong. The strong bond of carbon double bonded to oxygen is found in amino
acids (these will be discussed later). The number of valence electrons shared
also controls the "shape" of the atomic interactions. Carbon double bonded to
oxygen forms a "flat" (planar) bond that does not rotate. This limits the shapes
that the larger macromolecule, with repetitive double bonds, can form.
Formation of Hydrogen Molecule
 A covalent bond is formed between two hydrogen atoms.
A covalent bond is formed between two hydrogen atoms.
Formation of Methane and Ammonia Molecules

Polar Covalent Bonds
In a molecule such as hydrogen, the electrons are shared equally because each
atom has the same electronegativity. However, in some molecules one atom is more
electronegative than another, in which case the electrons are not shared
equally. For example, in a water molecule, one oxygen atom is covalently
bonded
to two hydrogen atoms. Because an oxygen atom is more electronegative than a
hydrogen atom, the oxygen atom draws the electrons being shared toward itself
and away from the less electronegative hydrogen. We say that the bond between
the oxygen and hydrogen is polar because the electrons are shared unequally.
This unequal sharing of electrons results in the more electronegative element,
in this example the oxygen atom, having a slightly negative charge and the less
electronegative element, in this example the hydrogen atom, having a slightly
positive charge. Molecules with polar bonds have characteristics of both ionic
and covalent bonds. Whether or not a molecule is polar has significant
implications on how that molecule interacts with other molecules and ions in
biological systems.
Water is the basis of life. Without it, life is not possible. Water accounts for up to 75
percent of the weight of the human body. Water provides a relatively stable medium in
which chemical reactions can take place. Water’s unique chemical properties (as a
solvent with a high boiling point and high heat capacity) make it essential for
homeostasis. The body’s thermoregulation relies on water’s high heat capacity to buffer
it against swings in external temperature. Cooling of the body is carried out by
evaporative water loss—perspiration. Water is also vital as a transport medium. Oxygen
is carried by red blood cells suspended in serum, which is mostly water. Nutritional
substances are dissolved in water and transported to cells. Water is used to dissolve
and dilute waste molecules. If the body is deprived of water for very long, death will
result.
Water has several properties that contribute to its suitability to support life as we
know it. One of these properties is that water is a polar molecule. Oxygen
is more electronegative than hydrogen and draws the electrons that it shares in the
covalent bond towards itself. Because water is polar, the partial positive end of one
water molecule will be attracted to the partial negative end of a neighboring water
molecule. This attraction is called a hydrogen bond. occurs between partially negatively charged atoms with high
electronegativity—oxygen, nitrogen or fluorine—and partially positively charged hydrogen
atoms that are bonded to oxygen, nitrogen or fluorine atoms.
Hydrogen bonding, which is referred to as an intermolecular attraction, is a critical
interaction within the cell. It is the principal force that holds the tertiary structure
of proteins, carbohydrates and nucleic acids together and the overall stability of these
molecules is due in part to the cumulative effect of the large number of hydrogen bonds
found in the functional structures. Hydrogen bonds are found in and between a variety of
molecules. For example, the enormous number of hydrogen bonds between strands of plant
cellulose provide the strength and structure of the plant cell wall. As another example,
wool (sheep hair) has lots of proteins with an enormous number of hydrogen bonds that
provide the curly structure of individual wool fibers. These curly fibers trap air
spaces which makes wool such a good insulator. When washed at high temperatures, these
hydrogen bonds are broken and the wool fibers will lose their shape, probably damaging
any wool clothing.
The electronegative oxygen (red) draws electrons to it, creating the partial
negative charge on oxygen and partial positive charge on hydrogen.
Adjust the volume on your computer and click the play button. Click on topic
(partial charges) to see other animation choices.
A water molecule attracts four other water molecules towards
itself. One molecule to each of the two free pairs of electrons in the
oxygen atom valence shell, and one to each of the hydrogen atoms
covalently bonded to the oxygen. As the water molecules associate with
each other
they have a defined structure dictated by the tetrahedral geometry of
the electrons
around the oxygen atom as seen in the figure below. The partially
positive hydrogen
atoms are attracted to the free electron pairs from other water
molecules while the
partially negative charge on the oxygen's free electron pairs are
attracted to the
partially positive hydrogen atoms from another water molecule.
Example
Click and drag to rotate the ice lattice. Use the right mouse button on the
PC, or control click inside the FlashMol to control zooming to get a better
look.
One excellent illustration of the hydrogen bonding is the change in hydrogen bonding
of water in ice. These stabilized bonds give ice its crystal-like appearance. Use
your mouse on the ice lattice above to explore the network-like formation of weak
bonds between molecules. This type of hydrogen bonding also helps large protein
structures stabilize.
Water and Ions
The attraction between oppositely charged ions in an ionic compound is strong.
However, because of the polarity of water, when many ionic compounds are in
aqueous solutions they become dissociated into ions. For instance, when an ionic
compound such as table salt (NaCl) is dissolved in water, it separates into
Na+
and Cl- ions. The water molecules surround the
ions to form polar interactions such that the positive ends of the water
molecules are arranged around negative ions and the negative ends of the water
molecules surround positive ions. Thus the ions become encapsulated by water
spheres, which are called spheres of hydration. The
biological world is very ionic, and spheres of hydration are important in a cell
because they maintain the separation of the many ions of the cell from each
other. The sphere of hydration must be broken in order for binding to take place
with a specific binding partner.
Hydrophilic Interaction
The nature of polar molecules is that they contain highly electronegative atoms.
Consequently, many are capable of hydrogen bonding with aqueous or polar
solvents. Because polar molecules are generally water soluble, they are referred
to as being hydrophilic, or water-loving. The one-carbon alcohol,
methanol, is an example of a polar molecule.
Hydrophobic Interaction
The final type of interaction occurs between neutral hydrophobic, or
water-fearing, molecules. These nonpolar molecules do not interact with water
and are characterized by atoms with the same or nearly the same
electronegativities.
In aqueous
solutions,
the hydrophobic molecules are driven together to the exclusion of water. For
example, shaking a bottle of oil and vinegar (acetic acid in water), such as in
a salad dressing, results in the oil being dispersed as tiny droplets in the
vinegar. As the mixture settles, the oil collects in larger and larger drops
until it only exists as a layer, or phase, above the vinegar.
A similar effect occurs in biological systems. As a protein folds to its final
three-dimensional structure, the hydrophobic parts of the protein are forced
together and away from the aqueous environment of the cell. Similarly,
biological membranes are stabilized by the exclusion of water between layers of
lipids as we will see later.
Amphipathic molecules are molecules that have a distinct nonpolar, or
hydrophobic, region, and a distinct polar region. These molecules do not form
true solutions in water. Rather, the nonpolar parts are forced together into a
nonpolar aggregate, leaving the polar part of the molecule to interact with the
aqueous phase. Detergents and long-chain carboxylic acids are examples of
amphipathic molecules.
Energy Associated with the Bonds
Each of the bond types represents a measurable amount of energy. To break a
bond, the equivalent amount of energy must be expended. In metabolism, bonds are
broken in molecules, such as glucose, to "release" the energy. The cell utilizes
this energy to drive other energy-consuming reactions. The covalent bond has the
most energy associated with it, on average approximately 100 kilocalories/mole
(kcal/mol). The noncovalent bonds, ionic and hydrogen, and hydrophobic
interactions, have approximately five kcal/mol associated with each of them.
It should be noted here that throughout the presentation of this course
approximations will be used for certain values so that estimations can be made
as we move to more complex systems. It is to be acknowledged that very precise
values for each of the measurements
are
not available.
Thus the noncovalent bonds that have been introduced have approximately 20 times
less energy associated with them and, thus, are more easily broken individually.
However, hydrogen bonds generally form extensive networks, and the total energy
associated with the network is the sum of the individual interactions (van der
Waals force). As anyone who has done a "belly flop" into a swimming pool knows,
breaking a large surface area of water is extremely difficult (and painful).
| Energy associated with the different bonds |
|---|
| Bond |
Energy, kcal/mol |
| Covalent |
100 |
| Ionic |
5 |
| Hydrogen |
5 |
| Hydrophobic interactions |
5 |
| van der Waals |
5 (depends on surface area) |
|
When NaCl dissolves in water, each ion becomes surrounded by at least 20 water
molecules. As NaCl there is 5 kcal/mol of energy associated with the ionic
attraction of the cation and anion, but when a Na ion is surrounded by 20 water
molecules, there is 100 kcal/mol of energy associated with just the Na ion.
Thus, NaCl in an aqueous solution is energetically more favored than NaCl as the
ionically bonded molecule due to the resulting hydrated state. You will explore
what happens to molecules that only partially dissociate in water, or weak
electrolytes, in the next module.
Electrolytes
An electrolyte is any fluid that contains free ions. You have already learned about ions and
ionic properties. Important ions in physiology include sodium, potassium,
calcium, chloride and phosphate. These ions are used in maintaining protein
structure and in cell communication, and generally can help maintain water
balances throughout the body. We get electrolytes through ingestion. Drinks with
“electrolytes” have salts (sodium and potassium) that help maintain ion
homeostasis for athletes that sweat and lose salt. Electrolytes are essential
for life, but many people get too much (like too much sodium from salt in
processed food), which can also disrupt proper physiological function.
pH
The hydrogen ion concentration (H+) of a solution is an important property,
because biological systems contain functional groups whose properties are
changed by changes in the hydrogen ion concentration.
Since the hydrogen ion concentrations are usually much less than one, and can vary over many
orders of magnitude, a different scale is used to describe the hydrogen ion
concentration—the pH scale. The pH is the negative logarithm (-log) of the
proton concentration:
The log
conversion reduces a tenfold change in hydrogen ion concentration to a one unit
change in pH. The minus sign changes the negative numbers that would be obtained
from log(H
+) to positive ones. Since the pH scale is an inverse
scale, the concentration of protons is high at low pH and low at high pH. A
solution is said to be acidic if the pH is less than 7.0, and basic if the pH is
more than 7.0. A solution is neutral if its pH is equal to 7.0.
The image below shows the pH of a number of common fluids.
pH of various
compounds
 On the left are biological compounds and on the right are some foods and cleaning products.
On the left are biological compounds and on the right are some foods and cleaning products.
Acid Dissociation and pH
For our studies, the Bronsted definition of an acid will be used. Here, we will define an
acid as a proton donor and a base as a
proton acceptor. Hydrochloric acid, like sodium chloride, is a strong
electrolyte because it completely dissociates in aqueous solution into charged
ions. Hydrochloric acid is also a strong
acid,
because when it completely dissociates it also completely donates all of its
protons.
Many molecules are weak electrolytes and exist in an equilibrium (indicated by in the general equation below) between the starting molecule and its dissociated parts. Thus dissociation can be seen as an acid (HA) in equilibrium with a proton (H+) and the corresponding conjugate base (A-).
In general:
Specifically for acetic acid:
The simplest of the macromolecules are carbohydrates, also called saccharides. The name is
descriptive of the character of this class of molecules, since they all have the
general formula of a hydrated carbon.
This represents a 2:1 ratio of hydrogen to oxygen atoms(as in water). But the constituent
atoms of carbohydrates can be configured in virtually endless configurations, so
carbohydrate molecules come in a multitude of different shapes and sizes.
Monosaccharides are the most basic units of carbohydrates. These are simple sugars, including
glucose, fructose, and others. They contain between three and seven carbon
atoms, have a sweet taste and are used by the body for energy.
Polysaccharides are long polymers of monosaccharide sugars that are covalently bonded
together. Polysaccharides are often used to store the energy of the
monosaccharide. These include starch and glycogen. Polysaccharides can also be
used for structure in plants and other lower organisms. For example, cellulose
is a large polysaccharide that is found in plant cell walls. People can't digest
cellulose into monosaccharides, but it is important in our diets as "roughage"
or "insoluble fiber."
Polysaccharides can be conjugated with other macromolecules. For example, complex
carbohydrates can be linked with proteins or lipids to form glycoproteins and
glycolipids, respectively. Very different structures can be made from a few
monosaccharides arranged in different patterns and with different bonding. This
flexibility in structure can therefore be used for identification of individual
cell types, since the structure of each cell type is unique. More than half of
the proteins in the body, which we will discuss later in this module, have
glycosylations or carbohydrate modifications. The outside of cells are covered
in carbohydrates from modifications of lipids that make up the membrane; we will
cover lipids in the last chapter of this section.
Carbohydrates are best know as energy storage molecules. Their primary function is as a
source of energy. Cells readily convert carbohydrates to usable energy. You will
recall that molecules are a collection of atoms connected by covalent bonds. In
general, single covalent bonds can be represented as having approximately 100
kcal/mol of energy associated with the force that holds the two atoms together.
Table sugar, or sucrose, is the best-known carbohydrate. The most common
carbohydrate in nature is glucose, which has the general formula
and which is a common
source of energy for many living organisms. If glucose is completely metabolized
or burned for its energy in a cell, the complete breakdown of the molecule
follows the following equation:
While the overall reaction represents a coupled oxidation/reduction process, on balance this
process involves the breaking of five carbon-to-carbon bonds with the release of
673 kcal/mol of energy.
However, the body does not need dietary carbohydrates for energy. Proteins and fats can meet
the body's needs, and the body can convert molecules into carbohydrates needed
for other cellular functions. But carbohydrates require minimal processing for
use as energy. For example, a simple enzymatic reaction converts sucrose into
blood sugar, which can be used directly as a source of cellular energy. The
trick for the cell is to convert the 673 kcal of energy to a useful form so that
it can do work for the cell or organism. The metabolic fate of the carbohydrate
will be discussed later in the course.
A second function performed by carbohydrates is structure. In this case, structure is not
only what a polymer of carbohydrates has, but also what that structure
contributes to the cell. For example, cellulose is a linear polymer of glucose
that interacts with other cellulose polymers to form fibers that interact to
form the basic structure of the cell wall of plants. These cellulose polymers
are undigestable and constitute the roughage

Another example is the peptidoglycan that makes up the cell wall structure of a bacterium.
"Peptido" refers to a peptide, which is a fragment of a protein or a short
polymer of amino acids, and "glycan" refers to a polysaccharide, a polymer of
carbohydrates. In this case, the polymer of carbohydrates includes building
blocks other than glucose, but the end result is the formation of a matrix. In
both cases, the resulting fibers and matrices provide scaffolding that gives
rigidity (structure) and protection to the bacteria. Gram staining used to
identify bacteria is based on the staining property of this peptidoglycan and
some antibiotics work by destroying this protective layer so bacteria are killed
by water rushing into the cell by osmosis.
Carbohydrates are also the structural basis for nucleic acids, which we will cover later.
A third function of carbohydrates is cell recognition and signaling.
Just as a peptidoglycan is a conjunction (conjugation) of a peptide with a
polysaccharide, other complex carbohydrates are conjugated to other molecules to
form glycoproteins (carbohydrates linked to proteins) and glycolipids
(carbohydrates linked to lipids). Because a very large number of structures can
be made from a few monosaccharides (simple carbohydrates), a very large number
of different structures can also be made from a few simple carbohydrates, as
will be seen later. This large number of different structures can therefore be
used for identification of individual cell types.
Carbohydrate modifications (called glycosylations) are present on lipid membranes and
proteins for specialized function and recognition. Unique carbohydrate
formations
allow even more specificity to a protein, beyond just the amino acid code. The
outer membrane of the cell is dotted with carbohydrate chains, which differ
according to cell type. These carbohydrate glycosylations provide a "signature"
of the cell and can also act as a signal. Thus, glycosylations are important in
immune response and general cell-to-cell communication.
After nucleic acids, proteins are the most important macromolecules. Structurally,
proteins are the most complex macromolecules. A protein is a linear molecule comprised
of amino acids. Twenty different amino acids are found in proteins. The sequence of a
protein's amino acids is determined by the sequence of bases in the DNA coding for the
synthesis of this protein. A single protein molecule may be comprised of hundreds of
amino acids. This sequence of amino acids is a protein's primary
structure. The protein's size, shape and reactive properties
depend on the number, type and sequence of amino acids. The amino acid chain can remain
in its primary linear structure, but often it folds up and in on itself to form a shape.
This Secondary
structure forms from localized interactions (hydrogen bonding) of
amino acid side chains. These include alpha helix and beta sheet structures. The alpha
helix is dominant in hemoglobin, which facilitates transport of oxygen in blood.
Secondary structures are integrated along with twists and kinks into a three-dimensional
protein. This functional form is called the tertiary
structure of the protein. An additional level of organization
results when several separate proteins combine to form a protein complex—called quaternary
structure.

Proteins perform numerous essential functions within the cell. Many proteins serve as
enzymes, which control the rate of chemical reactions, and hence the responsiveness of
cells to external stimuli. An enzyme can fast-forward a reaction that would take
millions of years under normal conditions and make it happen in just a few milliseconds.
Enzymes are important in DNA replication, transcription and repair. Digestive processes
are also largely facilitated by enzymes, which break down molecules that would otherwise
be too large to be absorbed by the intestines. Enzymatic proteins also play a role in
generation and transmission of nerve impulses, for example helping to generate muscle
contractions.
Other proteins are important in cell signaling and cell recognition. Receptor proteins
recognize substances as foreign and initiate an immune response. Through cell signaling,
proteins mediate cell growth and differentiation during development. Several important
proteins provide mechanical support for the cell, scaffolding that helps the cell
maintain its shape. Other proteins comprise much of the body’s connective tissue and
structures such as hair and nails.
For protein production in cells the body needs amino acids, which we ingest. It seems a
bit inefficient, but we eat proteins, break them down into amino acids, distribute the
amino acids inside the body and then build up new proteins. Our cells can synthesize
some amino acids from similar ones, but essential amino acids must be obtained from the
diet, since they cannot be synthesized. Deficiencies of protein in the diet result in
malnutrition diseases such as kwashiorkor, which is common in developing countries. In
cases of kwashiorkor, protein deficiency causes edema (swelling) which leads to a
distended abdomen. Excess protein is metabolized into ammonia and urea, which are
typically excreted—up to a point. When too much urea accumulates it cannot be filtered
by the kidneys. Urea accumulation can cause yellowing of the skin.
Unlike nucleic acids, which must remain unchanged in the body for the life of the
organism, proteins are meant to be transient—they are produced, do their functions and
then are recycled. Proteins are also readily broken down by extremes of heat or pH. When
you boil an egg, the yolk and white stiffen and change color. When you cook meat, the
flesh changes color and becomes firm. These changes arise because the constituent
proteins are “denatured” and have lost their secondary and tertiary structures.
Introduction
Amino acids are the building blocks of proteins. The sequence of amino acids in individual
proteins is encoded in the DNA of the cell. The physical and chemical properties
of the 20 different naturally occurring amino acids dictate the shape of the
protein and its interactions with its environment. Certain short sequences of
amino acids in the protein also dictate where the protein resides in the cell.
Proteins are composed of hundreds to thousands of amino acids. As you can
imagine, protein folding is a complicated process and there are many potential
shapes due to the large number of combinations of amino acids. By understanding
the properties of the amino acids you will gain an appreciation for the limits
of protein folding and will learn how to predict the potential higher-order
structure of the protein.
All amino acids have the same backbone structure, with an amino group (the -amino, or alpha-amino, group), a carboxyl group, an -hydrogen, and a variety of (R) all attached to the -carbon.
 The general structure of an -amino acid. The acidic group is a carboxylic acid. The carbon that
is attached to the carboxylic acid is the -carbon. If the R group were a carbon atom, it would be the -carbon.
The general structure of an -amino acid. The acidic group is a carboxylic acid. The carbon that
is attached to the carboxylic acid is the -carbon. If the R group were a carbon atom, it would be the -carbon.
If all of the amino acids have the same basic structure with an amino, a carboxyl and a
hydrogen fixed to the alpha-carbon, then the large variation in the properties
and structure of the amino acids must come from the fourth group attached to the
alpha carbon. This group is referred to as the side chain of the amino
acid or the R group.
The structures of the 20 common amino acids are shown on the chart below. The simplest amino
acid, glycine, is shown in the upper left. The main-chain atoms of glycine are
highlighted in yellow and its side chain (H) is highlighted in green. All amino
acids have the same main-chain atoms, but differ in the side chains. For
clarity, the -proton is omitted in the remaining drawings. Nonpolar amino acids are
highlighted in gray, aromatic amino acids are highlighted in cyan, polar are
highlighted in purple, amino acids with acidic side chains are highlighted in
red, and amino acids with basic side chains are highlighted in blue. The amino
acids cysteine and proline, which are shown at the bottom of the chart, have
unique properties. Cysteine can form a covalent S-S disulfide bond, stabilizing
the protein structure. In the case of proline, the side chain attaches to the
nitrogen, making proline an imino acid (rather than an amino acid).
The side-chain groups of these amino acids contain many of the same functional groups that
were discussed earlier in this unit and that can be found in the functional
groups interactive chart, which can be accessed by clicking on the Learn by
Doing link below.
Peptide Bonds
Proteins are polymers of amino acids. The amino acids are joined together by a
condensation
reaction. Each amino acid in the polymer is referred to as a
"residue." Individual amino acids are joined together by the attachment of the
nitrogen of an amino group of one amino acid to the carbonyl carbon (C=O) of the
carboxyl group of another amino acid, to create a covalent peptide bond and
yield a molecule of water, as shown below.
 Peptide bond formation occurs by a dehydration reaction. The amino group of the second
amino acid attaches to the carbonyl carbon of the first, forming the peptide
bond and releasing water. The resultant dipeptide has an amino terminus
(left) and a carboxy
terminus
(right). The
main-chain
atoms, which are the same for each residue in the peptide, include the
nitrogen and its proton, the -carbon and its hydrogen, and the
C=O
group. The R
groups
form the
side-chain
atoms.
Peptide bond formation occurs by a dehydration reaction. The amino group of the second
amino acid attaches to the carbonyl carbon of the first, forming the peptide
bond and releasing water. The resultant dipeptide has an amino terminus
(left) and a carboxy
terminus
(right). The
main-chain
atoms, which are the same for each residue in the peptide, include the
nitrogen and its proton, the -carbon and its hydrogen, and the
C=O
group. The R
groups
form the
side-chain
atoms.
The resulting peptide chain is linear with defined ends. Short polymers (less than 50
residues or amino acids) are usually referred to as peptides, and longer
polymers or polypeptides. Several polypeptides together can form some large
proteins. Because the synthesis takes place from the alpha-amino group of one
amino acid to the carboxyl group of another amino acid, the result is that there
will always be a free amino group on one end of the growing polymer (the
N-terminus) and a free carboxyl group on the other end (the C-terminus). Note
that the potential exists for the formation of amide (peptide) links involving
the carboxyl and amino groups in the side chains, but
bioselectivity
directs the synthesis to be linear, involving only the alpha-amino and
alpha-carboxyl groups.
Note that after the amino acid has been incorporated into the protein, the charges on the
amino and carboxy termini have disappeared, thus the main-chain atoms have
become polar functional groups. Since each residue in a protein has exactly the
same main-chain atoms, the functional properties of a protein must arise from
the different side-chain groups.
By convention, the sequences of peptides and proteins are written with the
N-terminus on the left and the C-terminus on the right. The name of the
N-terminal residue is always the first amino acid. The name of each amino acid
then follows. The primary sequence of a protein refers to its amino
acid sequence.
Primarily located in the cell nucleus (hence the name) are replicating
macromolecules. The most important are DNA and RNA. Without them, cells could not
replicate; life would not be possible. These molecules store the cell’s “software”—the
instructions that govern its function, processes and structure. The code is comprised of
sequences of four bases—adenine, cytosine, guanine and thymine (uracil in RNA). These
are arranged in sets of three called triplets. Each triplet specifies an amino acid,
which in turn is a component of a protein macromolecule. All the intricate complexity of
the human body arises from the information encoded by just four chemicals in a single
long DNA macromolecule.
In humans, mistakes in the structures of DNA and RNA cause diseases, including sickle
cell anemia, hemophilia, Huntingdon's chorea and some types of cancer. Even a small
error can result in a dramatic effect. Sickle cell disease is caused when just one amino
acid in the DNA base sequence is changed. Through directing chemical processes, nucleic
acids instruct cells how to differentiate into various organs. During development, whole
sets of DNA sequences are shut down or activated to drive specific processes. These
processes lead to different kinds of cells that form organs such as the heart, liver,
skin and brain.
Within the cell, nucleic acids are in turn organized into higher-level structures called
chromosomes. You can see chromosomes with a light microscope, using an appropriate
stain. Early study of chromosomes helped scientists discover and understand the role of
nucleic acids in cellular reproduction. Errors in chromosomal structure lead to
malfunctions of life processes. For example, in humans, an extra chromosome 21 results
in Down Syndrome.
The Backbone
 Structure of RNA and DNA
Structure of RNA and DNA
Our genetic code is determined by only four bases in DNA (G, C, A, T), which are
repeated and arranged in a special order. For example,
1 agccctccag gacaggctgc atcagaagag gccatcaagc agatcactgt ccttctgcca
61 tggccctgtg gatgcgcctc ctgcccctgc tggcgctgct ggccctctgg ggacctgacc
121 cagccgcagc ctttgtgaac caacacctgt gcggctcaca cctggtggaa gctctctacc
181 tagtgtgcgg ggaacgaggc ttcttctaca cacccaagac ccgccgggag gcagaggacc
241 tgcaggtggg gcaggtggag ctgggcgggg gccctggtgc aggcagcctg cagcccttgg
301 ccctggaggg gtccctgcag aagcgtggca ttgtggaaca atgctgtacc agcatctgct
361 ccctctacca gctggagaac tactgcaact agacgcagcc cgcaggcagc cccacacccg
421 ccgcctcctg caccgagaga gatggaataa agcccttgaa ccagcaaaa
This may seem like a random string of G, C, A, T, but this DNA codes for human
insulin. DNA is organized into a linear polymer in a double helix and maintains
the inherited order of bases or genetic code. The "steps" of the DNA ladder have
the code that ultimately directs the synthesis of our proteins. This linear
polymer of genetic code is maintained when double strand DNA is transcribed to
single strand RNA.
 Structure of a nucleotide
Structure of a nucleotide
The fundamental unit of DNA is the nucleotide. The nucleotide contains a
phosphate group (shown in orange), which will eventually give the DNA polymer
its charge and interconnect nucleotides on the backbone. The furanose sugar
group is a five-sided sugar (shown in purple). The nitrogenous base (shown in
yellow) gives the DNA its specificity.
The numbering of the positions on the sugar furanose rings of DNA and RNA follow
a convention that uses ' (the prime symbol) to denote the sugar positions. Thus,
the ribose has a nitrogenous base connected to the 1' position and hydroxyl
groups (OH) on the 2', 3' and 5' positions. Using this nomenclature, deoxyribose
is formally called 2'-deoxyribose (2 prime deoxyribose) to denote the loss of
the hydroxyl at the 2' position of ribose.
The major difference in the polymer backbones between DNA and RNA is the sugar
used in the formation of the polymer. In DNA (DeoxyriboNucleic
Acid) the 2' position of the furanose has a hydrogen. In RNA
(RiboNucleic Acid), the 2' position of the
furanose has an OH (hydroxyl) and the sugar is the monosaccharide ribose in the
furanose conformation.
 Furanose sugars
Furanose sugars
The linkage of individual nucleotides is made by a bridging phosphate molecule
between two hydroxyl groups, one on each furanose ring. The resulting polymer is
a string of furanose molecules linked by phosphodiester bonds in one very long
macromolecule.
 Backbone of DNA
Backbone of DNA
The following is a list of structural characteristics of the DNA/RNA polymer
backbone.
- Phosphate-ribose(deoxyribose)-phosphate-ribose(deoxyribose) sequence
- Linked by Phosphodiester covalent bonds
- 3' position on one ribose(deoxyribose) linked to 5' position of adjacent
ribose(deoxyribose) through phosphodiester bridge
- Chain has 3' end and 5' end
Hydrogen Bonding Between Bases
The DNA double helix is held in place with the hydrogen bonding of purines to
pyrimidines. Recall that hydrogen bonds are weak interactions, not like the
covalent bonds of the phosphate-furanose backbone. Thus, DNA is held together,
but can be pulled apart for transcription to RNA or for DNA replication.
To maintain equal distance between the two strands of DNA, the larger purines
must bind with the smaller pyrimidines. Specifically, A always binds with T and
G always binds with C in DNA. A useful memory device is that A and T are angular
letters and G and C are both curvy.
The following is an
interactive
simulation that allows you to form hydrogen bonding pairs
between the appropriate bases in DNA and RNA that would be allowed in the
formation of the double helical structure. You should apply the definition of
the hydrogen bond to form all possible hydrogen bonds in any pair of bases you
choose. All of the possible hydrogen bonds may be useful later as we explore
multiple structures, especially for RNA. The focus of this exercise is to
identify bonding partners that will be optimal in the formation of the DNA and
RNA helical structures.
Select the proper base
from the right to bond with the example on the
left. Draw the hydrogen bonds between then and
then click the Done when you have drawn all of the bonds and then
identify the bases.
DNA Transcription
DNA replication: Every time a cell divides, all of the DNA of the genome
is duplicated (called replication) so that each cell after the division (called
a daughter cell) has the same DNA as the original cell (called the mother cell).
DNA transcription: For the genetic code to become a
protein, it goes through a transcription step. DNA is transcribed into RNA (a
single-strand nucleic acid). The RNA is then shuttled away from the DNA to the
region of protein synthesis.
RNA translation: RNA is translated from a nucleic acid code
into the amino acid sequence of a protein.
Thus, the DNA gene code is able to duplicate to maintain consistency throughout
the person's body and throughout the person's life. DNA is also used to make
proteins through the use of an RNA intermediate.
Lipids include fats and waxes. Several vitamins, such as A, D, E and K, are lipid
soluble. Perhaps the most important role of lipids is in forming the membranes of cells
and organelles. In this way, lipids enable isolation and control of chemical processes.
They also play a role in energy storage and cell signaling.
Lipid molecules forming cell membranes are comprised of a hydrophilic “head” and
hydrophobic “tail” (remember, "hydro" means water and "philos" means love; "hydro"
means water, "phobic" means fear). A phospholipid bilayer is formed when the two layers
of phospholipid molecules organize with the hydrophobic tails meeting in the middle.
Scientists believe that the formation of cell-like globules of lipids was a vital
precursor to the origin of cellular life, since membranes physically separate
intracellular components from the extracellular environment. Thus, lipid membranes
enclose other macromolecules, confine volumes to increase the possibility of reaction,
and protect chemical processes. Proteins with hydrophobic regions float within the lipid
bilayer. These molecules govern transport of charged or lipophobic molecules in and out
of the cell, such as energy molecules and waste products. Carbohydrate molecules jutting
out of the membrane are important for cell recognition as mentioned previously.
Lipids are also vital energy storage molecules. Carbohydrates can be used right away, and
lipids provide long-term energy storage. Lipids accumulate in adipose cells (fat cells)
in the body. As part of the
catabolic
process, from the days when humans had to forage for food, excess carbohydrates can be
converted into lipids, which are then stored in fatty tissue. Ultimately, too many
ingested carbohydrates and lipids lead to obesity.
Cells are the basis of life—the basic structural unit of living things. Molecules such as
water and amino acids are not alive but cells are! All life is comprised of cells of one
type or another.
One of the hallmarks of living systems is the ability to maintain
homeostasis, or a relatively constant internal state. The cell is the
first level of complexity able to maintain homeostasis, and it is the unique structure
of the cell that enables this critical function.
In this section of the course, you will learn about the cell and all the parts that make
it functional. You will also focus on the cell membrane, which is the structure that
surrounds the cell and separates its internal environment from the external environment.
It is a critical component because it controls what can enter and exit the cell. This
section will also describe how cells reproduce to maintain homeostasis.
The current cell theory states that:
- All known living things are composed of one or more cells.
- All new cells are created by pre-existing cells dividing in two.
- The cell is the most basic unit of structure and function in all living
organisms.
Modern cell theorists assert that all functions essential to life occur within the cell;
and that, during cell division, the cell contains and transmits to the next generation
the information necessary to conduct and regulate cell functioning.
Let’s begin our study of the cell by investigating the basic anatomy of an animal cell.
Each cell consists of three components shown in the image above.
- A cell membrane which surrounds and protects the cell
- The cytoplasm which is the watery interior of the cell which contains ions,
proteins, and organelles
- Organelles which carry out all activities necessary for the cell to live, grow, and
reproduce
Within the body, cells represent a level of organization between organelles and tissues.
Organelles in turn are comprised of specialized macromolecules and tissues are
collections of specialized cells. Brain, kidney, liver, muscle and lung tissues differ
from each other because of the structure and function of their constituent cells. Thus,
the cells comprising each tissue type vary in shape, size and interior structure to
permit their specific physiological function within the tissue.One important concept to
keep in mind as you study anatomy and physiology is that structure determines function.
When you look at the shape of a cell, it gives you a clue as to it’s function.
did I get this
Observe the cells below and think about how the shape is necessary for its role.
See if you can match the cell with its function.
Each cell process is carried out in a specific location in the cell, often located in or
around an organelle. Think of an organelle as a level of organization
between macromolecules and the cell. Organelles carry out specialized tasks within the
cell, localizing functions such as replication, energy production, protein synthesis,
and processing of food and waste. The various cells differ in the arrangement and number
of organelles, as well as structurally, giving rise to the hundreds of cell types found
in the body.
The focus of this section is to understand the organelles of the cell, how they interact
with each other, and how they function during transport, growth and division in the
cell. You will learn about the controlled chemical environment a cell maintains and what
restrictions this places on the types of chemical reactions it can perform. This
background is vital to understanding key processes such as how a cell releases energy
from glucose, makes and folds proteins, and goes through growth and cell division.
Think of a city and the various jobs within a city. A cell is similar with each organelle
serving a specific purpose. There are organelles whose job is to provide shape and
structure to the cell, much like the city streets and bridges. These protein rich
organelles include intermediate filaments, microtubules, and
microfilaments. Some of these actually move other organelles around the
cell or change the shape of the cell. When a muscle cell contracts or shortens it does
so by the microfilaments made up of the proteins actin and myosin. One special organelle
composed of microtubules is located in an area near the nucleus, the
centrosome. The centrosome contains a pair called of microtubule
bundles known as the centrioles. Centrioles are important because they move
chromosomes to opposite ends of the cell during cell replication termed mitosis. Neurons
do not have centrioles and cannot replicate.

Other organelles help synthesize the proteins needed by the cell. These protein factories
are called ribosomes. They can be scattered within the cell or attached to
a membrane channel system called the endoplasmic reticulum or ER. When the
ER has ribosomes attached to it, it is termed the rough ER (the ribosomes give it a
rough or grainy appearance). When the ER lacks ribosomes it is termed the smooth ER and
functions for lipid synthesis and storage of toxins. When a protein is manufactured it
must be folded into a specific shape to work. Often additional side chains of
carbohydrates must be attached. The protein is processed in the rough ER. Once it is
formed it enters the golgi apparatus which is the distributing plant for
the cell. It completes any protein processing and then packages it into a
vesicle for transport to its destination. Some proteins are needed in
the cell membrane and the vesicles make sure they reach the membrane. The golgi
apparatus also makes a special type of vesicle termed a lysosome. The
lysosome is the garbage man of the cell. It takes in cell debris and waste and destroys
it. The lysosome contains very powerful hydrolytic enzymes to accomplish this. It is
very important that the enzymes remain in the lysosome or they would destroy the
cell.
The power plant of the cell is the mitochondria. This organelle generates
the ATP or energy for the cell. Mitochondria even have their own DNA termed
mitochondrial DNA (mDNA) and can replicate.
Finally there is the controller of the cell. This is the nucleus. Not all
cells have a nucleus and are termed anucleate. If you look at the image of the red blood
cells you will see a white dot in the center of the cell – that is where the nucleus
used to be. The nucleus is ejected when they mature. Some cells have more than one
nucleus and are termed multinucleate. Skeletal muscle cells are very large
cells and are multinucleate. The nucleus contains the DNA of the cell and the nucleolus.
The nucleolus is an organelle that makes ribosomes. The DNA is your genetic
code. It contains the genes that contain the instructions for making every protein in
your body. The nucleus is surrounded by it’s own membrane with tiny holes termed
nuclear pores. The membrane is called the nuclear membrane or
nuclear envelope.
The interactive diagram below shows a drawing of a eukaryotic cell. The cell components
in the list link to images that highlight these same structures in a living cell.
learn by doing
Practice what you have learned by following the link and answering the questions
in the interactive diagram.
The cell membrane is a dynamic structure composed of lipids, proteins, and carbohydrates.
It protects the cell by preventing materials from leaking out, controls what can enter
or leave through the membrane, provides a binding site for hormones and other chemicals,
and serves as an identification card for the immune system to distinguish between “self”
and “non-self” cells. We will first investigate the anatomy of the cell membrane and
then continue on to study the physiology of membrane transport.
The phospholipid bilayer is the main fabric of the membrane. The bilayer's
structure causes the membrane to be semi-permeable. Remember that phospholipid molecules
are amphiphilic, which means that they contain both a nonpolar and polar region.
Phospholipids have a polar head (it contains a charged phosphate group) with two
nonpolar hydrophobic fatty acid tails. The tails of the phospholipids face each other in
the core of the membrane while each polar head lies on the outside and inside of the
cell. Having the polar heads oriented toward the external and internal sides of the
membrane attracts other polar molecules to the cell membrane. The hydrophobic core
blocks the diffusion of hydrophilic ions and polar molecules. Small
hydrophobic molecules and gases, which can dissolve in the membrane's
core, cross it with ease.
Other molecules require proteins to transport them across the membrane.
Proteins determine most of the membrane's specific functions. The
plasma membrane and the membranes of the various organelles each have unique collections
of proteins. For example, to date more than 50 kinds of proteins have been found in the
plasma membrane of red blood cells.

Importance of Phospholipid Membrane Structure
What is important about the structure of a phospholipid membrane? First, it is
fluid. This allows cells to change shape, permitting growth and movement. The
fluidity of the membrane is regulated by the types of phospholipids and the
presence of cholesterol. Second, the phospholipid membrane is selectively
permeable.
The ability of a molecule to pass through the membrane depends on its polarity
and to some extent its size. Many non-polar molecules such as oxygen, carbon
dioxide, and small hydrocarbons can flow easily through cell membranes. This
feature of membranes is very important because hemoglobin, the protein that
carries oxygen in our blood, is contained within red blood cells. Oxygen must be
able to freely cross the membrane so that hemoglobin can get fully loaded with
oxygen in our lungs, and deliver it effectively to our tissues. Most polar
substances are stopped by a cell membrane, except perhaps for small polar
compounds like the one carbon alcohol, methanol. Glucose is too large to pass
through the membrane unassisted and a special transporter protein ferries it
across. One type of diabetes is caused by misregulation of the glucose
transporter. This decreases the ability of glucose to enter the cell and results
in high blood glucose levels. Charged ions, such as sodium (Na+) or potassium
(K+) ions seldom go through a membrane, consequently they also need special
transporter molecules to pass through the membrane. The inability of Na+ and K+
to pass through the membrane allows the cell to regulate the concentrations of
these ions on the inside or outside of the cell. The conduction of electrical
signals in your neurons is based on the ability of cells to control Na+ and K+
levels.
Selectively permeable membranes allow cells to keep the chemistry of the
cytoplasm different from that of the external environment. It also allows them
to maintain chemically unique conditions inside their organelles.
The cell membrane is not a static structure. It is a dynamic structure that allows the
movement of phospholipids and proteins. Fluidity is a term used to describe the ease of
movement of molecules in the membrane and is an important characteristic for cell
function. Fluidity is dependent on the temperature (increased temperatures it more fluid
and decreased temperatures make it more solid), saturated fatty acids and unsaturated
fatty acids. Saturated fatty acids make the membrane less fluid while unsaturated fatty
acids make it more fluid. The correct ratio of saturated to unsaturated fatty acids
keeps the membrane fluid at any temperature conducive to life. For example, winter wheat
responds to decreasing temperatures by increasing the amount of unsaturated fatty acids
in cell membranes to prevent the cell membrane from becoming too solid in the cold. In
animal cells, cholesterol helps to prevent the packing of fatty acid tails and thus
lowers the requirement of unsaturated fatty acids. This helps maintain the fluid nature
of the cell membrane without it becoming too liquid at body temperature.
The fluidity of the membrane is demonstrated in the
animation
below. You will want to click the green arrow when the animation pauses to see all three
parts. Select the magnifying glass to enlarge image.
learn by doing
Click the green arrow to play the animation.
Membranes also contain proteins, which carry out many of the functions of the membrane.
Some functions of membrane proteins are:
-
Transport. Because the plasma membrane is only semipermeable, the cell
needs a way to transport larger materials into and out of the cell.
-
Communication. Because the plasma membrane is the border of the cell, this
is where the cell communicates with its environment. Membrane proteins are able to
receive signals from outside the cell and begin a chain of events that cause the
cell to respond to these signals.
-
Metabolism. Membrane proteins can be enzymes that are involved in the
chemical reactions of metabolism. These are the processes that allow the cell to
grow, obtain energy, and eliminate wastes.
-
Adhesion. Membrane proteins help cells bind to each other and form tissues.
One example of this is skin cells, which must form a tight surface if the skin is to
maintain proper integrity. Membrane proteins also bind to molecules inside and
outside the cell which help the cell maintain its structure.
Membrane proteins are classified into two major categories: integral proteins and
peripheral proteins. Integral membrane proteins are those proteins that are
embedded in the lipid bilayer and are generally characterized by their solubility in
nonpolar, hydrophobic solvents. Transmembrane proteins are examples of
integral proteins with hydrophobic regions that completely span the hydrophobic interior
of the membrane. The parts of the protein exposed to the interior and exterior of the
cell are hydrophilic. Integral proteins can serve as pores that selectively allow ions
or nutrients and wastes into or out of the cell. They can also transmit signals across
the membrane.
Unlike integral proteins that span the membrane, peripheral proteins reside
on only one side of the membrane and are often attached to integral proteins. Some
peripheral proteins serve as anchor points for the cytoskeleton or extracellular fibers.
Proteins are much larger than lipids and move more slowly. Some move in a seemingly
directed manner, while others drift. Some are glycoproteins which have a carbohydrate
group attached to the protein. These are on the outside of the membrane and important
for cell recognition, they work like a cellular identification card.
The extracellular surface of the cell membrane is
decorated
with carbohydrate groups attached to lipids and proteins. Carbohydrates are added to
lipids and proteins by a process called glycosylation, and are called glycolipids or
glycoproteins. These short carbohydrates, or oligosaccharides, are usually chains of 15
or fewer sugar molecules. Oligosaccharides give a cell identity (i.e., distinguishing
"self" from "nonself") and are the distinguishing factor in human blood types and
transplant rejection.
Membranes are Asymmetric
As discussed above and seen in the picture, the cell membrane is asymmetric. The extracellular
face of the membrane is in contact with the extracellular matrix. The
extracellular side of the membrane contains oligosaccharides that distinguish
the cell as "self." It also contains the end of integral proteins that interact
with signals from other cells and sense the extracellular environment. The inner
membrane is in contact with the contents of the cell. This side of the membrane
anchors to the cytoskeleton and contains the end of integral proteins that relay
signals received on the external side.
Summary: Membranes as Mosaics of Structure and Function
The biological membrane is a collage of many different proteins embedded in the fluid matrix
of the lipid bilayer. The lipid bilayer is the main fabric of the membrane, and
its structure creates a semipermeable membrane. The hydrophobic core impedes the
diffusion of hydrophilic structures such as ions and polar molecules, but allows
hydrophobic molecules, which can dissolve in the membrane, to cross it with
ease.
Diffusion relies on kinetic energy and a concentration gradient. Kinetic energy is
affected by temperature, size of molecules, steepness of the gradient, and the medium
the molecules are in. Anything that increases the kinetic energy of the molecules will
increase the rate of diffusion.
Diffusion is the movement of molecules from an area of
high concentration to an area of lower concentration. There are different types of
diffusion: simple in which the molecule passes directly through the phospholipid bilayer
and facilitated which uses the integral membrane proteins as channels. Simple diffusion
occurs when the molecules are either very small or lipid soluble and pass through the
phospholipid bilayer of the cell membrane. Some examples of substances that use this
process are oxygen (O2), carbon dioxide (CO2), and lipids.
The molecules will move from an area of high concentration down its diffusion (or
concentration) gradient to the lower concentration until equilibrium is reached. Once
the concentration is the same on both sides of the membrane, the molecules continue to
move but they maintain the same levels at equilibrium.
Have you ever had the pleasant surprise of waking up to the smell of coffee or
bacon? It’s the diffusion of the chemicals (odorants) moving from the higher
concentration in your kitchen to you! In the human body the diffusion of O2 and CO2 are
critical for gas exchange. O2 levels are higher in your arterial blood than your tissue
cells so O2 will diffuse out of the blood into your cells. CO2 has the opposite
concentration gradient. CO2 levels are highest in your cells (your mitochondria produce
CO2 as a waste product from cellular respiration) and CO2 diffuses out of the cell into
the blood. These molecules are small enough to pass through the phospholipid bilayer and
are examples of simple diffusion. Remember that anything that increases kinetic energy
will also increase the rate of diffusion.
The following animation
depicts this simple diffusion process.
Diffusion Across the Membrane
Molecules can be divided into four categories with regard to their ability to cross the
plasma membrane. The first category is
nonpolar molecules. These hydrophobic
molecules can easily cross the membrane because they
interact favorably with the nonpolar lipids. Note that these molecules can
accumulate in the membrane because they interact so well with the
lipids. The second category is small
polar molecules. Although they don’t interact with the
lipids, their small size allows them to pass through small temporary holes in
the membrane. The third category is large polar molecules.
These have difficulty crossing the membrane because of their size and poor
interaction with the lipids. The last category is ionic
compounds. Their charge interacts very unfavorably with the
lipids, making it very difficult for them to cross the membrane.
learn by doing
As three different molecules move, they encounter the lipid bilayer depicted
by the horizontal membrane across the center of the stage in the preceding
animation. Notice that one type of molecule passes freely through the lipid
bilayer while the second type of molecule only occasionally passes through
the membrane, and the lipid bilayer is totally impermeable to the third type
of molecule.
The size, polarity, and charge of a substance will determine whether or not the substance
can cross the cell membrane by diffusion. The cholesterol was an example of a lipid, and
is highly soluble in the nonpolar environment of the lipid bilayer. You saw, in the
animation above, the cholesterol freely passing into the hydrophobic environment of the
membrane. The cholesterol distributes freely in the membrane and then some fraction will
dissolve in the aqueous environment of the cytoplasm. Water, on the other hand, while
polar, is small and because of this is able to freely cross the membrane. The lipid
bilayer is much less permeable to the ion, because of its charge and larger size. As a
general rule, charged molecules are much less permeable to the lipid bilayer.
Cells must be able to move large polar and charged molecules across the lipid bilayer of the
membrane in order to carry out life processes. To allow these molecules, which are not
soluble in the lipid bilayer, to pass across the hydrophobic barrier it is necessary to
provide ports, channels or holes through the membrane. The molecules will still move
spontaneously down a concentration gradient from high to low concentration. Some of
these channels can remain open at all times, allowing the molecules to move freely
according to the concentration gradient. Others can be gated channels that open and
close in response to the needs of the cell. In most cases these channels are very
discriminatory and will only allow specific molecules to pass. The process of moving
impermeable molecules across a membrane (down their concentration gradients) using
channels or pores is referred to as facilitated diffusion. Because the molecules are
moving down a concentration gradient, the process is driven by simple diffusion and does
not require the input of additional energy from the cell. The following simulation
depicts the facilitated diffusion of glucose across the membrane using the glucose
permease transporter.
Cells continually encounter changes in their external environment. Most cells have a similar
blend of solutes within them, but interstitial or extracellular fluid can vary. What
will happen if there is a strong concentration gradient between a cell's interior and
the fluid outside? As you know, molecules will tend to move down their concentration
gradients until equilibrium is reached. You might think that solutes will flow into our
out of the cell until the solute concentrations are equal across the membrane. However,
not all molecules can pass through the cell membrane. The plasma membrane (lipid
bilayer) is significantly less permeable to most solutes than it is to water. Therefore
the WATER tends to flow in a way that establishes an equal concentration of solutes on
either side of the membrane. The water flows down its own concentration gradient, with a
net movement toward the region that has a higher concentration of solutes. This movement
of water across a semipermeable membrane in response to an imbalance of solute is called
osmosis.
The relationship between the solute concentration and amount of water is an inverse
relationship. The more concentrated a solution is, the less water it contains. The fewer
solutes, the more water – i.e. it is more dilute. Water follows gradients and moves from
an area of more water to less water but in reality water is moved to the area with the
greater number of solutes. This creates a pressure termed osmotic pressure. Cells cannot
actively move water, it must follow osmotic gradients. Solutions that have a greater
solute concentration will pull water via osmotic pressure. This depends on the total
number of solutes, not the type. Note that some water can pass through the cell membrane
but most water passes through protein membrane channels termed aquaporins
Cells may find themselves in three different sorts of solutions.
The terms isotonic,
hypertonic, and hypotonic refer to the concentration of solutes
outside the cell
relative to the solute concentration inside the cell. In an isotonic
solution, solutes
and water are equally concentrated within and outside the cell. 0.9%
NaCl (physiologic saline) and 5% dextrose are two common isotonic
solutions used.The cell is bathed in a
solution with a solute concentration that is similar to its own
cytoplasm. Many medical
preparations (saline solutions for nasal sprays, eye drops, and
intravenous drugs) are
designed to be isotonic to our cells. A hypotonic solution has a low
solute
concentration and a high concentration of water compared to the
cell's cytoplasm.
Distilled (pure) water is the ultimate hypotonic solution. If a cell
is placed in a
hypotonic solution, it will tend to gain water. The solutes will
"stay put" within the
cell, but water molecules will diffuse such that their net flow is
toward the area with
a higher concentration of solutes. A hypertonic solution has a high
solute concentration
(lower water concentration) compared to the cell cytoplasm. Very
salty or sugary
solutions (brines or syrups) are hypertonic to living cells. If a
cell is placed in such
a solution, water tends to flow spontaneously out of the cell.
Filtration is another passive process of moving material through a cell membrane. While
diffusion and osmosis rely on concentration gradients, filtration uses a pressure
gradient. Molecules will move from an area of higher pressure to an area of lower
pressure. Filtration is non-specific. This means that it doesn’t sort the molecules,
they pass due to pressure gradients and their size. If molecules are small enough to
pass through the membrane, they will. The force that pushes the molecules is termed
hydrostatic pressure.
Example
One example of filtration is making coffee. Think of the coffee
filter as the cell membrane and the coffee grounds, flavor and caffeine as the
molecules. The pressure is exerted by the water from the machine. It forces materials
through the coffee filter into the coffee pot. Small molecules like caffeine, water, and
flavor pass through the filter but the coffee grounds do not. They are too big. If you
poked holes in the filter, the coffee grounds would end up in your coffee! The coffee
filter represents the filtration membrane which is typically a layer of cells.
Filtration is one of the main methods used for capillary exchange.
Blood pressure
provides the driving force or hydrostatic pressure to force materials
out of capillaries
to cells or to form the filtrate (fluid in the nephron of the
kidney). Hydrostatic
pressure is countered by osmotic pressure. Remember osmotic pressure
is created due to
increased solute concentration and will pull water toward the area of
higher solutes.
These two pressures must be in balance for homeostasis of fluid
volumes. In our body large molecules such as plasma proteins and red
blood cells should not pass out of the blood through the cell membranes
lining the capillaries. If they pass through and end up in in the
tissues or in the kidney and later the urine it is abnormal and a sign
of disease.
You have just finished investigating the passive methods of transport, now let’s look at active
methods. In active methods the cell must expend energy (ATP) to do the work of moving
molecules. Active transport often occurs when the molecule is being moved against its
concentration gradient or when moving very large molecules into our out of the cell.
There are 3 main types of active processes.
- Primary Active Transport or Solute Pumps
- Endocytosis and Exocytosis
Primary Active Transport or Solute Pumps
One form of active transport involves moving ions from an area of low concentration to an
area of higher concentration. If you need to roll a rock it’s much easier to
roll it downhill rather than uphill right? Active transport is moving the rock
uphill and requires energy to do so. The best example of this involves the
sodium (Na+)/potassium (K+) exchange pump. The interior of a cell contains a low
Na+ concentration compared to the extracellular fluid. The interior of the cell
(intracellular fluid or ICF) contains more K+ than the extracellular fluid
(ECF). This creates an imbalance in these ions. Na+ has a concentration gradient
to enter the cell since there is more Na+ in the ECF. K+ has a concentration
gradient for it to leave the cell. The cell membrane is not impermeable to these
ions and some of them escape following their concentration or diffusion
gradients. You will later study why it is important to maintain these ion
concentrations. The Na+/K+ pump is an active transport pump that moves Na+
“uphill” back out of the cell against it’s concentration gradient and at the
same time moves K+ back into the cell against it’s concentration gradient. This
pump requires ATP, a membrane protein transporter, and enzymes to function.
Another form of active transport is termed secondary active transport. While primary active
transport directly uses ATP, secondary active transport relies on the energy from
electrochemical gradients to move molecules against their gradients. Primary active
transport sets this up because it actively pumps ions such as Na+ out of the cell
thereby creating an electrochemical gradient for Na+ across the cell membrane. Protein
transporters in the cell membrane use the energy from electrochemical gradients to
transport molecules. If the molecules move in the same direction this is known as
cotransport or symport. If they are moved in opposite directions this is known as
countertransport or antiport. One common occurrence in the human body is the Na+/glucose
transport protein known as SGLT1 which cotransports 1 glucose and 2 sodium ions into a
cell. The electrochemical gradient for Na+ into the cell allows glucose to ride with it.
When glucose moves into a cell, it rides the energy from the “coat tails of sodium
ions”.
Facilitated diffusion and active transport are not the only ways that materials can enter
or leave cells. Through the processes of endocytosis and exocytosis, materials can be
taken up or ejected in bulk, without passing through the cell's plasma membrane.
In endocytosis, material is engulfed within an infolding of the plasma
membrane and then brought into the cell within a cytoplasmic vesicle. To begin
endocytosis, a particle encounters the cell surface and produces a dimple or pit in the
membrane. The pit deepens, invaginates further, and finally pinches off to form a
vesicle in the cytoplasm of the cell. Note that during the process the inside surface of
the newly formed vesicle is the same as the exterior surface of the cell. Thus the
integrity of the cytoplasm and the orientation of the plasma membrane are preserved.
Once internalized, this new vesicle containing extracellular materials may fuse with a
lysosome so that its solid contents are digested. The resulting molecules may be
released to the cytoplasm for use within the cell.
There are two general forms of endocytosis: phagocytosis and
pinocytosis. Phagocytosis is the uptake of large solid
particles such as bacteria or cellular debris. Pinocytosis is the uptake of
fluid and any small molecules dissolved within it. Cells are also capable of recognizing
specific particles and engulfing them in a more targeted way, a process called
receptor-mediated endocytosis. In this case, the particle first binds to a membrane
protein receptor on the surface of the cell. Binding of the target particle induces the
cell to engulf it.
Exocytosis is just the reverse of endocytosis. In exocytosis, an internal
vesicle fuses with the plasma membrane and releases its contents to the outside. The
balance of exocytosis and endocytosis preserves the size of the plasma membrane and
keeps the cell's size constant. The following animation depicts endocytosis.
You can use the magnifier in the lower righ-hand corner to zoom the
animation
How are endocytosis and exocytosis important to everyday life? Immune cells protect
animals by recognizing and destroying foreign objects such as bacteria. Disease-causing
bacteria are recognized by proteins called receptors on the surface of the immune cell.
The phagocytic immune cell will then engulf the bacterial cells (phagocytosis). The
vesicle that contains these bacterial cells is called a phagosome ("phago" means
"eating" and "-some" refers to "body"). The phagosome next fuses with lysosomes.
Finally, the digested bacterial products are excreted through the process of
exocytosis.
Phagocytosis of a Bacterium

Even though we may not feel any changes from day to day, we are constantly repairing and
replacing cells within our bodies. It’s estimated that approximately 300 million cells
die every minute in our body! These cells must be replaced by identical functional cells
for us to survive and maintain homeostasis. The process of cell proliferation or cell
division is termed mitosis. Mitosis produces two daughter cells that are identical
(clones) to the parent cell.
Cell division is a carefully regulated process. There are “check-points” between phases,
in which the cell “self-checks.” Through molecular interactions, the cell makes sure
that it is ready to divide; for example, it checks to see that its DNA has not been
damaged. If all criteria are met, the cell moves forward through the cell cycle. Normal
cells usually divide only a limited number of times, and usually do so when stimulated
by certain molecular signals. For example, if you move to higher altitude, your body
will produce more red blood cells in order to supply enough oxygen despite the thinner
air.
Mitosis is also regulated by genes. Some genes become active to stimulate mitosis and
other genes act as the brakes to turn it off (suppressor genes). If our body loses
control over mitosis the result would be the production of too many (hyperplasia)
abnormal cells and would form a tumor. If the tumor is encapsulated it is termed benign.
If not, the mutated cells can spread and it is known as cancer. Cancer cells, on the
other hand, have lost the normal cell cycle controls, and therefore can divide
indefinitely.
When a cell is performing its normal day-to-day functions it is in a state termed
interphase. When it is time for that cell to replicate, genes become active and
stimulate it to begin mitosis. Mitosis is characterized by four phases. Remember if a
cell is going to form 2 new identical cells it must first replicate it’s DNA and
organelles. It is during interphase that the DNA is replicated to form 2 identical
strands. These strands of identical DNA are termed chromatids and are held together with
a centromere.
The stages of mitosis occur in sequence with specific events in each one. It is only
during mitosis that chromosomes are visible. Usually DNA is in its threadlike chromatin
form. You can identify the stages of mitosis by observing the chromosomes. Remember that
the DNA is in the nucleus which is surrounded by the nuclear membrane. The DNA needs to
be free from the nucleus so it can be evenly distributed to two daughter cells. The
events of mitosis describe the processes of splitting and moving nuclear DNA to opposite
ends of the parent cell where the nuclear membranes will reform. Then the cell membrane
can split the cytoplasm and organelles (termed cytokinesis). The two daughter cells will
each have the same genetic code. When you studied the cell you learned about the
microtubules of the centrioles. They are the organelles that will move the DNA during
mitosis.
The major stages of mitosis are:
- Prophase
- Metaphase
- Anaphase
- Telophase
Prophase
Mitosis begins when DNA condenses into chromosomes visible under a light
microscope. This packaging of the chromosomes into condensed bundles makes them
easier to sort, but it inhibits protein synthesis. Therefore, it is essential
that the cell has produced all necessary proteins prior to the start of this
process.
The nucleoli disappear and the nuclear envelope begins to disintegrate.
This allows cellular components to act upon the chromosomes (the chromosomes are
no longer tucked away inside the nucleus).
Centrioles, contained in the centrosomes formed during interphase, are
areas where microtubules originate in order to help sort and organize the sister
chromatids. The centrioles begin to move to the opposite poles of the cell. This
will determine where the chromosomes go when they are sorted.
Microtubules are disassembled from the cytoplasm and reassembled into
the mitotic spindle. This structure will move the chromosomes to the proper
location.
This stage is called “prophase,” because it is a preparatory stage.
“Pro-” means "before;" “phase” means “stage.” So, this is the stage before the
process gets into full swing.
Prometaphase
Prometaphase is the stage between prophase and metaphase. During this stage, the
nuclear envelope is fully broken down. This allows the microtubules to attach to
the centromeres of the chromosomes.
The centromere is the region in the center of the X-shaped chromosomes. Each half
of the X is a copy of the same DNA strand. The centromere contains proteins that
hold together these two copies and can bind to the microtubules. The proteins on
the centromere are called the kinetochore, and the microtubules that attach to
them are called kinetochore microtubules. The microtubules that are not attached
to the kinetochores are called nonkinetochore microtubules.
Metaphase
Metaphase is so named because the chromosomes line up in the middle of the cell.
The root “meta-” means “middle.”
The kinetochore microtubules are used to orient the chromosomes in the center of
the cell. Each chromosome will be attached to two kinetochore microtubules. Each
of these kinetochore microtubules will be attached to one of the two
centrioles.
Anaphase
During anaphase, the kinetochore proteins break down the microtubules attached to
them and the connections between each copy of the chromosome will be broken
down. This causes the individual chromosomes to move to opposite poles of the
cell. The root “ana-” refers to “apart”; the chromosomes are moving apart from
each other.
The nonkinetochore microtubules from one pole also push on the nonkinetochore
microtubules from the other pole. This causes the cell to elongate. By the end
of anaphase, each pole of the cell contains an identical set of chromosomes.
Telophase
In telophase, the nuclei at each pole form again. The chromosomes are now
separated into two identical nuclei. This is the end of mitosis.
However, the two nuclei are still in a single cell. The next step will separate
the cytoplasm into two cells. “Telo” comes from the Greek word for “end.”
Cytokinesis
Cytokinesis is the separation of the cytoplasm into two new daughter cells.
Animal cells divide when proteins pinch in the center of the cell until it
separates into two. This region is called the cleavage furrow.
Plant cells divide when new cell wall components are laid down in the center of
the cell. This is called the cell plate.
Apoptosis
How does a single fertilized cell develop into something as marvelous and
intricate as the human body? Part of the answer is apoptosis, or programmed cell
death. For example, the early hand of an embryo is a stubby appendage. As cells
between the fingers selectively die off, they sculpt the hand, leaving behind
the separate fingers. In adults, apoptosis is one way that tissues maintain
their integrity. Cells selectively die off to allow renewal of tissues such as
the stomach, lungs, liver and so on. If apoptosis ceases in the tissue’s cells,
a tumor can form, perhaps becoming cancerous. Apoptosis is distinguished from
necrosis, which is cell death as a result of injury. Much like proliferation,
there is a series of tightly regulated events that lead to apoptosis. Apoptosis
is a cellular program that is internally determined, and billions of cells die
this way every day. However, external trauma, toxins or infection cause a
different type of cell death called necrosis. Even though both mechanisms lead
to dead cells, apoptosis is a program to maintain cell number whereas necrosis
is a consequence of intense cell damage.
In the body's organizational hierarchy, tissues occupy a place between cells and organs.
That is, a tissue is a group of cells with a similar shape and function. In turn, organs
(which make up the body) are comprised of various tissues.
The component cells of a tissue are a specific cell type. A tissue’s cells may be
identical, but are not necessarily so. Several tissues will comprise an organ. For
example, the contractile cells of skeletal muscle are bundled together to make muscle
fiber tissue. In turn, endomysium cells form enclosing tissue that wraps around bundles
of muscle fibers, like a tortilla around the filling of a burrito. Several of these
structures are in turn wrapped by another tissue, perimysium. Finally, bundles of these
are surrounded by a sheath of yet another tissue, epimysium, which covers the outside of
the whole muscle. Yet more tissue is necessary for the muscle to function in the body.
Connective tissue comprising ligaments attaches the muscle to the skeleton, and nerve
tissue conducts impulses from the nervous system to signal the muscle to contract.
Skeletal muscle is only one kind of tissue. The body is made of dozens of different
tissues, but broadly speaking there are four types of tissues.
- Muscle tissue (in turn divided into skeletal, smooth and cardiac) is contractile. It
allows locomotion of the body. It also allows necessary contractions of various
organs such as the heart and of respiratory and digestive systems.
- Nerve tissue comprises the body’s wiring system. It conducts signals between the
nervous system and various organs.
- Connective tissue holds the body together. It is found in most organs, anchoring
them to the skeleton and other organs. Types of connective tissue include fibrous
tissue, fatty tissue, loose tissue and cartilage. Connective tissue also includes
bone, blood and lymph.
- Epithelial tissue is the body’s protection against the outside environment. Skin
tissue helps to maintain homeostasis. It helps monitor and control temperature, and
resists abrasion, foreign bodies and damaging chemicals. Internally, epithelial
tissue lines most internal cavities, secreting or absorbing
nutrients.

Tissues form during development. Stem cells in the embryo differentiate into various cell
types. The necessary genes in the cells turn on or off, resulting in the production of
proteins that characterize a cell’s structure and function. Early in embryonic growth,
the cells migrate to the appropriate location in the body. Once there, they proliferate
so that the tissue can perform its needed function.
Different tissues arise from the source cells in each of the three primary germ cell
layers. For example, the epithelium is derived from the ectoderm and endoderm.
Connective tissue arises largely from the mesoderm. Gastrointestinal and respiratory
tissues arise mostly from
the
endoderm. Programmed cell death, or apoptosis, may take place to
eliminate transitory tissues in the embryo, such as the pronephros, a simple excretory
organ that is later replaced by the kidney.
The organ level of organization in the body may be the most
familiar to us from our everyday experiences. Many of the common ailments we hear
about—an upset stomach, a broken bone, lung disease, skin cancer—are named for the
organs they affect.
An organ is made up of tissues that work together to perform a specific function
for the body as a whole. Groups of organs that perform related functions are
organized into organ systems, which perform more general functions. The table
below describes the structures and functions of some common organs.
| Organ |
Primary function(s)
|
Tissues it contains |
Organ system(s) it is a part of |
| brain |
control of body systems and behavior; cognition |
nervous, connective, epithelial |
nervous system; endocrine system |
| skin |
protection; support and containment; temperature and fluid
regulation |
epithelial, nervous,
connective,
muscular |
integumentary system |
| stomach |
chemical and mechanical digestion of food |
epithelial, connective, muscular, nervous |
digestive system |
| sternum (breastbone) |
support; protection; blood cell production |
epithelial,
connective,
nervous |
skeletal system; immune system; cardiovascular system |
| kidney |
waste removal; fluid regulation |
epithelial,
connective,
nervous |
urinary system |
Organ systems
are
made up of organs that work together to perform a specific function for the body as a
whole. The table below describes the organ
systems
and
their
primary organs and physiological functions.
| Organ system |
Key organ(s) |
Primary function(s) |
| integumentary |
skin |
support; protection; regulation of fluid levels and temperature |
| skeletal |
bones, cartilage |
support; protection; movement; blood cell production |
| muscular |
muscles, tendons |
support; movement |
| urinary |
kidneys, bladder, urethra |
waste removal; regulation of fluid levels |
| digestive |
tongue, esophagus, stomach, small intestine, large intestine, gallbladder,
rectum |
digestion of food; waste removal |
| respiratory |
trachea, lungs |
gas exchange; regulation of temperature |
| cardiovascular |
heart, blood vessels |
transport of materials through the body; regulation of temperature |
| nervous |
brain, spinal cord |
control of behavior and body systems; cognition |
| endocrine |
glands |
control of body systems and development |
| immune |
thymus, tonsils, spleen |
defense against infection |
| lymphatic |
lymph nodes, lymphatic vessels |
immunity; regulating fluid balance |
| reproductive |
penis, testes, prostate (males); uterus, ovaries, vagina (females) |
reproduction |
The Whole Body
The organ systems of the body all work together to maintain proper physiological
functions. Many times in
the
arena of anatomy and physiology, including in this course, we
closely
examine the molecules, cells, tissues and organs of the body
to learn their forms and functions. However, it is important to consider that
every molecule works as part of the entire system. Endocrine disorders such as
diabetes affect glucose levels in the body.
Altered
blood glucose levels can affect many organ systems. For example, the immune
system may not heal as well, the urinary system may experience kidney damage,
and the cardiovascular system can experience vascular damage, even to the point
of causing blindness. In the body, everything is interconnected.
Populations
Beyond the body, populations and environment can impact physiology and health.
Some diseases and disorders are common to certain populations, most likely
because
of
genetic connections. Also, environmental conditions can impact health.
Particulates in the air can impact respiratory function. We are also affected by
foods, exercise, sun
exposure
and other environmental conditions.
Homeostasis relates to dynamic physiological processes that help us maintain a stable
internal environment. Homeostasis is not the same as
equilibrium. Equilibrium occurs when everything is equal: add milk to the coffee and
eventually, when equilibrium is achieved, all of the coffee will be the same color.
Homeostasis, however, is the mechanism by which internal variables are kept at or near
values appropriate to the system.
Consider that when the temperature drops, the body does not just "equilibrate" with
(become the same as) the environment. Multiple systems work together to help maintain
the body’s temperature: we shiver, blood flow is altered, and our brain says “get out of
the cold.”
Many conditions and diseases result from altered homeostasis. This section will review
the terminology, and explain the physiological mechanisms, that are associated with
homeostasis. We will discuss homeostasis in every subsequent system. Many aspects of
the body are in a constant state of change—blood flows, that rate at which substances
are exchanged between cells and the environment, and the rate at which cells are
growing, dividing, and dying are all examples. But these changes actually contribute to
keeping many of the body's variables, and thus the body's overall internal conditions,
within relatively narrow ranges. For example, blood flow will increase to a tissue when
it becomes more active. This is done to ensure that the tissue will have enough oxygen
to support its higher level of metabolism. Maintaining internal conditions in the body
is called (from homeo-, meaning similar, and stasis, meaning standing still). The root
"stasis" of the term "homeostasis" may seem to imply that nothing is happening. But if
you think about anatomy and physiology, even standing still requires a lot of effort.
Stabilizing muscles hold you upright, and your brain incorporates information from your
tendons, inner ear and eyes to maintain balance. If you think that it’s easy to stand
still, look at a baby who is just learning to stand. Similarly, the process of
homeostasis is an active process to maintain the ‘same-ness’ of our body processes.
- homeostasis
-
(Definition)
Homeostasis is the tendency of biological systems to maintain relatively
constant conditions in the internal environment while continuously
interacting with and adjusting to changes originating within or outside the
system.
Example
Exercise
We can consider the maintenance of homeostasis on a number of different levels. For
example, consider what happens when you exercise. At the whole-body level, you
notice some specific changes: your breathing and heart rate increase, your skin may
flush, and you may sweat. If you continue to exercise, you may feel thirsty. These
effects are all the result of your body trying to maintain its internal balance:
- Your muscle cells use oxygen to convert the energy stored in glucose into the
energy stored in
ATP
(adenosine triphosphate), which they then use to drive muscle
contractions. When you exercise, your muscles need more oxygen. Therefore, to
maintain an adequate oxygen level in all of the tissues in your body, you
breathe more deeply and at a higher rate when you exercise. This allows you to
take in more oxygen. Your heart also pumps faster and harder, which allows it to
bring more oxygenated blood to your muscles and other organs that will need more
oxygen.
- As your muscles carry out cellular respiration to release the energy from
glucose, they produce carbon dioxide and water as waste products. These wastes
must be eliminated to help your body maintain its fluid and pH balance. Your
increased breathing and heart rates also help eliminate a great deal of carbon
dioxide and some of the excess water.
- Your muscles use the energy stored in ATP molecules to generate the force they
need to contract. A byproduct of releasing that energy is heat, so exercising
increases your body temperature. To maintain temperature balance, your body
compensates for the extra heat by causing blood vessels near your skin to dilate
and by causing sweat glands in your skin to release sweat. These actions allow
heat to more easily dissipate into the air through evaporation of the water in
sweat into the air.
- As you exercise for longer periods of time, you lose more and more water and
salts to sweat (and, to a smaller extent, from breathing more). If you exercise
too long, your body may lose enough water and salt that its other functions
begin to be affected. Low concentrations of water in the blood prompt the
release of hormones that make you feel thirsty.
Your
kidneys also remove less water from urine if your fluid levels are
low. These actions help you maintain fluid balance.
The maintenance of homeostasis in the body requires feedback loops that control the
body's internal conditions. We use the following terminology to describe feedback
loops:
-
Variables are parameters that are controlled or affected by the
feedback system.
-
Receptors detect changes in the variable.
-
Control centers (integrators) compare the variable in relation to a set
point and signal the effector to generate a corrective response.
-
Effectors execute the necessary changes to adjust the variable.

Air Conditioning
Air conditioning is a technological system that uses feedback. The thermostat
senses the temperature, an electronic interface compares the temperature against
a set point (the temperature that you want it to be). If the temperature matches
or is cooler, then nothing happens. If the temperature is too hot, then the
electronic interface triggers the air-conditioning unit to turn on. Once the
temperature is lowered sufficiently to reach the set point, the electronic
interface shuts the air-conditioning unit off. For this example, identify the
steps of the feedback loop.
Cruise Control
Cruise control is another technological feedback system. The idea of cruise
control is to maintain a constant speed in your car. The car's speed is
determined by the speedometer and an electronic interface measures the car's
speed against a set point chosen by the driver. If the speed is too slow, the
interface stimulates the engine; if the speed is too fast, the interface reduces
the power to the tires.
Example
Terms applied to temperature
Consider one of the feedback loops that controls body temperature.

-
Variable
In this instance, the variable is body temperature.
-
Receptors
Thermoreceptors detect changes in body temperature. For example,
thermoreceptors in your internal organs can detect a lowered body
temperature and produce nerve impulses that travel to the control center,
the hypothalamus.
-
Control Center
The hypothalamus controls a variety of effectors that respond to a decrease
in body temperature.
-
Effectors
There are several effectors controlled by the hypothalamus.
- blood vessels near the skin constrict, reducing blood flow (and the
resultant heat loss) to the skin.
- Skeletal muscles are also effectors in this feedback loop: they contract
rapidly in response to a decrease in body temperature. This shivering
helps to generate heat, which increases body temperature.
Remember that homeostasis is the maintenance of a relatively stable internal environment.
When a stimulus, or change in the environment, is present, feedback
loops respond to keep systems functioning near a set point, or
ideal level.
- feedback
-
(Definition)
Feedback is a situation when the output or response of a loop impacts or
influences the input or stimulus.

Typically, we divide feedback loops into two main types:
-
positive feedback loops, in which a change in a given direction causes
additional change in the same direction.
- For example, an increase in the concentration of a substance causes feedback
that produces continued increases in concentration.
-
negative feedback loops, in which a change in a given direction causes
change in the opposite direction.
- For example, an increase in the concentration of a substance causes feedback
that ultimately causes the concentration of the substance to
decrease.
Positive feedback loops are inherently unstable systems. Because a change in an input
causes responses that produce continued changes in the same direction, positive feedback
loops can lead to runaway conditions. Some positive feedback loops can be harmful.
However, there are a few instances in which positive feedback helps to maintain
homeostasis. One such example is blood clotting. When a blood vessel is broken, a clot
begins to form and clotting factors are activated at the site. The activation of
clotting factors continues into a chain reaction until bleeding within the vessel stops.
In this instance, the set point is the point at which no blood leaves the site. The
release of clotting factors stimulates further release of clotting factors, until the
blood is so heavily clotted that no blood leaves the vessels. Some positive feedback
loops are part of normal physiology. Some endocrine and reproductive functions,
particularly in women, are part of positive feedback loops.
Negative feedback loops are inherently stable systems. Negative feedback loops typically
produce conditions that oscillate around the set point. For example, negative feedback
loops involving insulin and glucagon help to keep blood glucose levels within a narrow
range of concentrations. If glucose levels get too high, the body releases insulin into
the bloodstream. Insulin causes the body's cells to take in and store glucose, lowering
the blood glucose concentration. If blood glucose gets too low, the body releases
glucagon, which causes the release of glucose from the body's cells.
In a positive feedback mechanism, the output of the system stimulates the system in such
a way as to further increase the output. Common terms that could describe positive
feedback loops include "snowballing" and "chain reaction". Without a counter-balancing
or "shut-down" reaction or process, a positive feedback mechanism has the potential to
produce a runaway process. Some physiological processes mediated by positive feedback
help maintain homeostasis. In these cases, the positive feedback loop always ends with
counter-signaling that suppresses the original stimulus.

Example
Blood clotting
Because of its ability to generate an amplifying process, beneficial positive
feedback is often seen where a rapid response is needed. For example, blood clotting
(or coagulation) starts with a wound that causes release of blood from damaged blood
vessels. When the lining of the blood vessels (endothelium) is ruptured, proteins in the
lining are exposed to collagen and other clotting factors. Platelets (small cell
fragments) in the blood stick to the edges of the blood vessels and form an initial
clot to plug the wound.
After they stick to the damaged blood vessels, the platelets release granules. The
granules contain additional stimulatory molecules including serotonin, adenosine
diphosphate (ADP), and thromboxane. The
ADP
attracts other platelets to the wound site. When they arrive, the thromboxane
prompts them to aggregate and to release more granules in turn. The ADP and
thromboxane thus promote the release of more ADP and more thromboxane in a positive
feedback cascade. The result is a platelet plug that closes the wound.
Following this first line of defense, multiple proteins called clotting factors act
together in a coagulation cascade to form a secondary clot. Once the bleeding stops,
the stimuli promoting clotting processes are no longer present, so the cascading
processes cease.
Example
Lactation
Another example of positive feedback occurs in lactation, during which a mother
produces milk for her infant. During pregnancy, levels of the hormone prolactin
increase. Prolactin normally stimulates milk production, but during pregnancy,
progesterone inhibits milk production. At birth, when the placenta is released from
the uterus, progesterone levels drop. As a result, milk production surges. As the
baby feeds, its suckling stimulates the breast, promoting further release of
prolactin, resulting in yet more milk production. This positive feedback ensures the
baby has sufficient milk during feeding. When the baby is weaned and no longer
nurses from the mother, stimulation ceases and prolactin in the mother’s blood
reverts to pre-breastfeeding levels.
Example
Heart failure
The above provide examples of beneficial positive feedback
mechanisms. However, in
many instances, positive feedback can be potentially
damaging to life processes. For
example, a myocardial infarction (heart attack) begins
when a small portion of heart
tissue dies off (usually due to inadequate blood
supply). The loss of tissue then results in too little blood being
pumped to the body tissues and cardiac muscle tissue, so the heart must
work harder and more heart tissue can become damaged and decrease heart
function further.

Most biological feedback systems are negative feedback systems. Negative
feedback occurs when a system's output acts to reduce or dampen the processes that lead
to the output of that system, resulting in less output. In general, negative feedback
loops allow systems to self-stabilize.

Example
Insulin
An important example is the control of blood sugar levels following a meal.
- After a meal, the small intestine absorbs glucose from digested food. Blood
glucose levels rise.
- Increased blood glucose levels stimulate beta cells in the pancreas to produce
insulin.
- Insulin triggers liver, muscle, and fat tissue cells to absorb glucose, where it
is stored. As glucose is absorbed, blood glucose levels fall.
- Once glucose levels drop below a threshold, there is no longer a sufficient
stimulus for insulin release, and the beta cells stop releasing insulin.

Due to synchronization of insulin release among the beta cells, basal insulin
concentration oscillates in the blood following a meal. The oscillations are
clinically important, since they are believed to help maintain sensitivity of
insulin receptors in target cells. This loss of sensitivity is the basis for insulin
resistance. Thus, failure of the negative feedback mechanism can result in high
blood glucose levels, which have a variety of negative health effects.
Example
Temperature
Negative feedback is a vital control mechanism for the body’s homeostasis. One
important example of how a negative feedback loop maintains homeostasis is the
body’s thermoregulation mechanism. The body maintains a relatively constant internal
temperature to optimize chemical processes. The hypothalamus, located in the brain,
monitors body temperature. Neural impulses from heat-sensitive thermoreceptors in
the skin signal the hypothalamus. As skin temperature rises, the hypothalamus
initiates release of water (sweat) from sweat glands. Evaporation cools the skin
until its temperature returns to normal. Once its temperature returns to normal, the
thermoreceptors in the skin cease to signal the hypothalamus, and sweating stops.
 Likewise, when body temperature drops, the hypothalamus initiates several
physiological responses to increase heat production and conserve heat:
Likewise, when body temperature drops, the hypothalamus initiates several
physiological responses to increase heat production and conserve heat:
- Narrowing of surface blood vessels (vasoconstriction) decreases the flow of heat
to the skin.
- Shivering commences, increasing production of heat by the muscles.
- Adrenal glands secrete stimulatory hormones such as norepinephrine and
epinephrine to increase metabolic rates and hence heat production.
These effects cause body temperature to increase. When it returns to normal, the
hypothalamus is no longer stimulated, and these effects cease.

Example
Blood Pressure Homeostasis
Many homeostatic mechanisms, like temperature, have different responses if the
variable is above or below the set point. When temperature increases, we sweat, when
it decreases, we shiver. These responses use different effectors to adjust the
variable. In other cases, a feedback loop will use the same effector to adjust the
variable back toward the set point, whether the initial change of the variable was
either above or below the set point. For example, pupillary diameter is adjusted to
make sure an appropriate amount of light is entering the eye. If the amount of light
is too low, the pupil dilates, if it is too high, the pupil constricts.
This might be compared to driving. If your speed is above the set point (the value you want it
to be), you can either just decrease the level of the accelerator (i.e. coast), or
you can active a second system -- the brake. In both cases you slow, but it can be
done by either just "backing" off on one system, or adding a second system.
Let’s look at how these two examples work related to normal blood pressure
homeostasis.
Let's take a closer look at diabetes. In particular, we will discuss diabetes
type 1 and type 2. Diabetes can be caused by too little insulin, resistance to insulin,
or both.
Diabetes Type 1 is usually diagnosed in childhood. However, many
patients are diagnosed when they are older than age 20. In this disease, the body makes
little or no insulin. Daily injections of insulin are needed. The exact cause is
unknown. Genetics, viruses, and autoimmune problems may play a role.
Also affected are those who lose their pancreas. Once the pancreas has been
removed (because of cancer, for example), diabetes type 1 is always present.
Diabetes Type 2 is far more common than type 1. It makes up most of
diabetes cases. It usually occurs in adulthood, but young people are increasingly being
diagnosed with this disease. The pancreas does not make enough insulin to keep blood
glucose levels normal, often because the body does not respond well to insulin. Many
people with type 2 diabetes do not know they have it, although it is a serious
condition. Type 2 diabetes is becoming more common due to increasing obesity and failure
to exercise.
You have read about general and specific examples of homeostasis, including positive and
negative feedback loops, and have learned the terminology that is used to describe parts
of the feedback loops. It is important to become comfortable with the terminology, since
it will be used to introduce new concepts in upcoming sections of this course.
Maintaining homeostasis within the body is important for proper physiological function,
growth and remodeling. It is important to recognize the mechanisms of homeostasis in the
body, as well as the consequences of homeostasis dysfunction.
In the following examples, you will learn to identify homeostasis at different levels of
organization, such as how the body maintains tight control over small molecules, and the
importance of maintaining cell number with regard to tissue homeostasis.
Body functions such as regulation of the heartbeat, contraction of muscles, activation of
enzymes, and cellular communication require tightly regulated calcium levels. Normally,
we get a lot of calcium from our diet. The small intestine absorbs calcium from digested
food.
The endocrine system is the control center for regulating blood calcium homeostasis. The
parathyroid and thyroid glands contain receptors that respond to levels of calcium in
the blood. In this feedback system, blood calcium level is the variable, because it
changes in response to the environment. Changes in blood calcium level have the
following effects:
- When blood calcium is low, the parathyroid gland secretes . This hormone causes effector organs (the kidneys and bones) to respond to
increase calcium levels. The kidneys prevent calcium from being excreted in the
urine. Osteoclasts in bones reabsorb bone tissue and release calcium.
- When blood calcium levels are high, the thyroid gland releases .Calcitonin causes the kidneys to reabsorb less calcium from the filtrate,
allowing excess calcium to be removed from the body in urine. Calcitonin also
suppresses the formation of active vitamin D in the kidneys; without vitamin D the
small intestines don't absorb as much dietary calcium. Osteoblasts, stimulated by
calcitonin, use calcium in the blood to add to bone tissue.
Calcium imbalance in the blood can lead to disease or even death.
Hypocalcemia refers to low blood calcium levels. Signs of hypocalcemia
include muscle spasms and heart malfunctions. Hypercalcemia occurs when blood
calcium levels are higher than normal. Hypercalcemia can also cause heart malfunction as
well as muscle weakness and kidney stones.
Glucose is an important energy source used by most cells in the body, especially muscles.
Without glucose, the body "starves," but if there is too much glucose, problems occur in
the kidneys, eyes, and even with the immune response. Insulin, a hormone produced by the
pancreas in response to increased blood glucose levels. When the pancreas releases
insulin, it acts as a key to open glucose passageways to enter cells. Glucose enters the
cells where it is used for energy production. Excess glucose is used to synthesize
glycogen for storage. The pancreas also produces the hormone glucagon. Glucagon is
released with blood glucose levels decrease and breaks down glycogen back to glucose
which is released into the blood to bring blood glucose levels back up.
Tissues have an optimal number of cells for function. Cells are able to interact with
their neighbors using cell-to-cell connections or cell-to-cell signaling to maintain
homeostasis of cell number. Normally cells will stop dividing when there is an
appropriate number of cells in a tissue or space. If a neighboring cell is lost or if
there is an inadequate number of cells, cells may be stimulated to divide. Cells with
too many neighbors trigger an internal response to die in a regulated programmed way
called apoptosis. When cells sense they have no neighbors, signals in the nucleus cause
division of the cell.
Each organ system performs specific functions for the body, and each organ system is
typically studied independently. However, the organ systems also work together to help
the body maintain homeostasis.
Example
Water Levels
For example, the cardiovascular, urinary, and lymphatic systems all help the body
control water balance. The cardiovascular and lymphatic systems transport fluids
throughout the body and help sense both solute and water levels and regulate
pressure. If the water level gets too high, the urinary system produces more dilute
urine (urine with a higher water content) to help eliminate the excess water. If the
water level gets too low, more concentrated urine is produced so that water is
conserved.
Example
Internal Temperatures
Similarly, the cardiovascular, integumentary, respiratory, and muscular systems work
together to help the body maintain a stable internal temperature. If body
temperature rises, blood vessels in the skin dilate, allowing more blood to flow
near the skin's surface. This allows heat to dissipate through the skin and into the
surrounding air. The skin may also produce sweat if the body gets too hot; when the
sweat evaporates, it helps to cool the body. Rapid breathing can also help the body
eliminate excess heat. Together, these responses to increased body temperature
explain why you sweat, pant, and become red in the face when you exercise hard.
(Heavy breathing during exercise is also one way the body gets more oxygen to your
muscles, and gets rid of the extra carbon dioxide produced by the muscles.)
Conversely, if your body is too cold, blood vessels in the skin contract, and blood
flow to the extremities (arms and legs) slows. Muscles contract and relax rapidly,
which generates heat to keep you warm. The hair on your skin rises, trapping more
air, which is a good insulator, near your skin. These responses to decreased body
temperature explain why you shiver, get "goose bumps," and have cold, pale
extremities when you are cold.
As you have learned, proper calcium levels are important to maintain whole body
homeostasis. Calcium ions are used for the heartbeat, the contraction of muscles, the
activation of enzymes, and cellular communication. The parathyroid and thyroid glands of
the endocrine system detect changes in blood calcium levels. When the parathyroid glands
detect low blood calcium levels, several organ systems alter their function to restore
blood calcium levels back to normal. The skeletal, urinary, and digestive systems all
act as effectors to achieve this goal through negative feedback.
The release of parathyroid hormone from the endocrine system triggers osteoclasts of the
skeletal system to resorb bone and release calcium into the blood. Similarly, this
hormone causes the kidneys of the urinary system to reabsorb calcium and return it to
the blood instead of excreting calcium into the urine. Through altered function of the
kidneys to form active vitamin D, the small intestine of the digestive system increases
the absorption of calcium.
When the thyroid gland detects elevated blood calcium levels, the skeletal, urinary, and
digestive systems contribute to lower blood calcium levels back to normal. Release of
the hormone calcitonin from the thyroid gland of the endocrine system triggers a series
of responses. The osteoblasts of the skeletal system use excess calcium in the blood to
deposit new bone. The kidneys of the urinary system excrete excess calcium into the
urine instead of reclaiming calcium through reabsorption. Lastly, the kidneys stop
forming active vitamin D, which causes decreased intestinal absorption of calcium
through the digestive system.
The endocrine functions of the pancreas and liver coordinate efforts to maintain normal
blood glucose levels. When pancreatic cells detect low blood glucose levels, the
pancreas synthesizes and secretes the hormone glucagon. Glucagon causes the liver to
convert the polymerized sugar glycogen into glucose through a process known as
glycogenolysis. Glucose then travels through the blood to allow all cells of the body to
use it.
If pancreatic cells detect high blood glucose levels, the pancreas synthesizes and
releases the hormone insulin. Insulin causes polymerization of glucose into glycogen,
which is then stored in the liver through a process known as glycogenesis.
The nervous and digestive systems also play a role in maintaining blood glucose levels.
When the stomach is empty and blood glucose levels are low, the digestive system and the
brain respond by making you feel hungry—your stomach may "growl," and you may feel pain
or discomfort in your midsection. These sensations prompt you to eat, which raises blood
glucose levels.
All organ systems require a balance of cell division and apoptosis during development, growth,
and repair to maintain tissue structure and function. The endocrine and immune systems are
important regulators for cell populations. The endocrine system delivers steroids and growth
hormones that send survival signals to specific tissues so that apoptosis is prevented.
Additionally, the endocrine system delivers some hormones that work to induce apoptosis under
some physiological conditions.
The cells of the immune system screen the blood for cells that divide at inappropriate times.
Immune cells produce antibodies to mark these out-of-control cells for destruction. A breakdown in
these processes can lead to the formation of tumors.
Like all body systems, the skeletal system can be affected by disease and injury. By
studying the responses of the skeletal system to these conditions, we can learn
about how the components of the skeletal system interact and how the skeletal system
interacts with the rest of the body. We will consider six common dysfunctions of the
skeletal system:
- Fractures: Breakage of the solid bones.
- Osteoarthritis: a chronic inflammation and damage of the articular cartilage.
- Osteogenesis imperfecta: A genetic condition leading to small bones.
- Osteomalacia ("rickets"): A bone disorder caused by a lack of vitamin D.
- Osteoporosis: A bone weakening condition, most common in women, caused by hormonal changes.
- Rheumatoid arthritis: An autoimmune disease that can damage the articular cartilage.
While the skeletal system primarily supports the body and allows movement, it
also performs a variety of important functions and impacts many other organ
systems.
Bones
Bones are the primary organs in the skeletal system. Their functions include:
- protection of vital structures, such as the spinal cord, brain, heart, and
lungs.
- support of body structures.
- body locomotion through coordination with the muscular system.
- hematopoiesis, or generation of blood cells, within the red marrow spaces of
bones.
- storage and release of the inorganic minerals calcium and phosphorous, which
are needed for functions such as muscle contraction and neural signal
conduction.
It's common to think of the skeletal system as being made up of only bones, but the
skeletal system contains many types of structures. In addition to localizing blood cell
formation and storing calcium, bones come together at locations called
articulations (or joints) to allow for locomotion and work. In any
complex system that moves (such as a bicycle or car), allowing functional, repetitive
motion requires a lot of control and support.
Connective Tissues
In addition to bones, the skeletal system contains several other important
connective tissues: , , and .
Cartilage is a firm yet pliable substance that performs a variety of functions:
protection, shape maintenance and support, lubrication, and shock absorption.
Its
primary function is to coat the end of the bones where they articulate with one
another,
providing a smooth, cushioned surface. Furthermore, cartilage can serve as a
template for bone formation during development and bone healing (this will be
further discussed in the section about bone homeostasis).

Ligaments and tendons support articulations and control the muscle attachment to
the bones. Ligaments connect bones to one another and stabilize articulations.
Tendons connect bones to muscles.

The structures of ligaments and tendons are similar, in that both tissues are
made of fibrous proteins aligned in the direction of force. The types of
proteins and differences in stretch and recoil distinguish the mechanical
behavior of ligaments and tendons. Ligaments are stiffer, but deform more, since
they stabilize articulations. Tendons are wrapped with a continuation of the
fascia that surrounds muscle cells to help transmit and dissipate force.
The adult human skeletal system is composed of 206 bones. The skeleton is divided into
two functional groupings—the
and the
.
The Axial Skeleton

The axial skeleton is composed of the skull, hyoid bone, vertebral column, and
the thorax (ribs and sternum). The axial skeleton functions to protect and
support organs of the head, neck, and trunk.
The skull consists of 22 facial and cranial bones that interlock to
form openings for the eyes and protection for the brain. The
cranium, a collection of bones that protect the brain, and the
mandible are the two major parts of the skull.
The hyoid bone is not attached to any other bone and is located in
the neck, and it supports tongue movement.
The vertebral
column consists of individual vertebrae separated by
cartilage disks. The vertebral column forms the middle axis of the skeleton.
The thoracic cage protects the internal organs of the upper abdomen.
The thoracic cage consists of the ribs and the sternum. The ribs articulate
anteriorly with the sternum and posteriorly with the vertebrae of the thorax.
The table below lists the location and function of the major bones of the axial
skeleton:
| Bone(s) |
Location |
Function |
Major grouping of axial skeleton |
| Cranium |
Head |
Supports facial structures, encloses and protects the brain, provides
muscle attachments for chewing and moving the head |
Skull |
| Mandible |
Lower jaw |
Permits chewing |
Skull |
| Vertebrae |
Spine |
Permit mechanical stability for the body and protect the spinal
cord |
Vertebral column |
| Ribs |
Chest wall |
Provide protection for the organs of the upper body |
Thoracic cage |
| Sternum |
Center of the chest |
Provides attachment for many (not all) ribs |
Thoracic cage |

The appendicular skeleton is composed of the upper limbs, lower limbs, pectoral
girdle, and pelvic girdle. The appendicular skeleton functions to anchor the
limbs to the axial skeleton.
The pectoral girdle consists of a scapula and clavicle on each
side of the body. The pectoral (shoulder) girdle permits movement of the upper limbs by
connecting the upper limbs to the axial skeleton.
The upper limbs of the appendicular skeleton are composed of
the humerus or upper arm bone, the radius and ulna, which complement each other to form
the forearm, and the wrist. The hand subdivides into smaller bones of the palm
and fingers.
The pelvic girdle of the appendicular skeleton is composed of two coxal
bones (fused ilium, ischium and pubis bones), which attach to the vertebral column and
the lower limbs.
The lower limbs each consist of the femur, or thigh bone; the tibia, or
shinbone and the fibula, or calf bone; and the foot. The patella is the bone located at
the point where the femur and tibia articulate with each other. The foot subdivides into
smaller bones of the ankle, instep, and toes.
The table below lists the location and function of the major bones of the
appendicular skeleton:
| Bone(s) |
Location |
Function |
Major grouping of appendicular skeleton |
| Scapula |
Flat, triangular bone located on the posterior side of each shoulder |
Articulates with the clavicle and humerus |
Pectoral girdle |
| Clavicle |
Located in each shoulder at the base of the neck |
Helps to keep the shoulders in place; connects upper arm to the body |
Pectoral girdle |
| Humerus |
Extends from the scapula to the elbow |
Provides attachments for muscles that move the shoulder and upper arm at the
proximal end; articulates with the radius and ulna at the distal end |
Upper limbs |
| Radius |
Located on the lateral side of the forearm between the elbow and wrist |
Provides attachment for muscles that bend the arm at the elbow and muscles that
allow movement of the wrist |
Upper limbs |
| Ulna |
Located on the medial side of the forearm between the elbow and wrist |
Provides attachment for muscles that bend and straighten the arm at the elbow
and muscles that allow movement of the wrist |
Upper limbs |
| Ilium |
Located on the superior portion of the coxal bone |
Connects the bones of the lower limbs to the axial skeleton |
Pelvic girdle |
| Femur |
Extends from the hip to the knee |
Provides attachment for muscles of the lower limbs and buttocks; distal end
articulates with the tibia and patella |
Lower limbs |
| Tibia |
Located on the medial side of the leg between the knee and the ankle |
Articulates with the femur, on its superior side, to form the knee joint;
articulates with the fibula on the lateral side; articulates with the patella on
the anterior side; and the tarsels to form the ankle joint |
Lower limbs |
| Fibula |
Located on the lateral side of the tibia between the knee and ankle |
Forms the lateral part of the ankle joint |
Lower limbs |
| Patella |
Located on the anterior surface of the articulation between the femur and
tibia |
Supports movement of the knee joint |
Lower limbs |

The bones of your body are mineralized structures that make up a major portion of
your skeleton. In the broad sense, a bone is composed of bone tissue, cartilage,
ligaments, tendons, vasculature, and nervous tissue. Bone tissue is a collection
of specialized cells (osteoblasts, osteoclasts, osteocytes), organic
extracellular matrix proteins (collagen and proteoglycans) and inorganic salt
crystals that work together to provide strength and flexibility.
Although all bones have a similar composition, their large-scale structures and functions
differ. One way to classify bone tissue is based on their microscopic structure.
- Bone tissue with a tightly packed microstructure arranged into rings is
called compact bone (also called cortical or lamellar bone).
Compact bone structure gives bones their stiffness. However, if bone structures were
made only of compact bone, they would be very heavy and brittle.
- Bone tissue with a porous microstructure is spongy
bone (also called cancellous or trabecular bone). Spongy bone consists of
branching bone structures called . Spongy bone helps to reduce the weight and brittleness of bone, and is
found at the ends of bone where forces are high. Spongy bone allows bones to bend a
slight amount without cracking.

Most bones of the body are composed of both spongy and compact bone tissues, so
that they are stiff, resilient and lightweight.
A common way to classify individual bones of the skeletal system is based on their
shapes. The table below describes the four main shapes of bones.
| Shape |
Description |
Examples |
| long bones |
significantly longer in one direction than in either of the other two
directions |
humerus (upper arm); femur (thigh) |
| short bones |
similar length in all directions |
most carpal (wrist) and tarsal (ankle) bones |
| flat bones |
flat and plate-like |
skull |
| irregular bones |
not regular or systematic in shape |
vertebrae; hip bones |
Components of Long Bones
A typical long bone, such as the femur, has three main components:
- the or shaft;
- the epiphyses (singular, ), found at the bone
ends;
- and the metaphyses (singular, metaphysis),
transitional areas between the diaphysis and epiphyses.
 Components of long bones. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Components of long bones. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The diaphysis or shaft is the longest part of a long bone. The epiphyses
(epiphysis if singular) are the ends of a long bone that articulate (connect)
with other bones at articulations or joints. The transitional area between the
diaphysis and epiphysis is the metaphysis (meta- means "in between"). The
medullary cavity, which houses yellow bone marrow, begins at
the boundary between the metaphysis and diaphysis of each bone end and runs
throughout the shaft or diaphysis of the bone. This marrow is rich in fat which
is why it is termed yellow bone marrow. The epiphyses contain
mainly spongy (cancellous) bone and red bone marrow. In children, the junction
between the epiphysis and metaphysis contains a layer of hyaline
cartilage,termed the epiphyseal plate. You have most probably heard
of this as the growth plate. This layer of cartilage is what allows bone to
continue to lengthen. The cartilage cells undero mitosis and move away from the
center of the bone toward the epiphyses while the bone grows into it and takes
over. In adult bone, this cartilage has become calcified and hard, the bone has
caught up and replaced all of the cartilage with bone. Now this area is termed
the epiphyseal line. The only area of hyaline cartilage left is on
the outer edge of each epiphysis. This cartilage is termed articular
cartilage and functions to cushion and reduce friction between bones
at articulations.

We can also use a bone's location within the axial or appendicular skeleton to
classify it. In addition to classifying bones based on their shape, we divide
the skeleton into axial and appendicular segments. The axial skeleton is
composed of the skull, the hyoid bone in the throat, the vertebral column, and
the thoracic cage. The appendicular skeleton is composed of the upper limbs,
lower limbs, pectoral girdle, and pelvic girdle.
It's common to think of the skeletal system as being made up of only bones, and
performing only the function of supporting the body. However, the skeletal system also
contains other structures, and performs a variety of functions for the body.
While the bones of the skeletal system are fascinating, it is our ability to move
segments of the skeleton in relation to one another that allows us to move around. Each
connection of bones is called an articulation or a joint. Articulations are classified based on material at the joint and the movement allowed at the joint.
Synarthrosis Articulations
Immovable articulations are articulations ("syn" means together and "arthrosis" means joint);
immovable articulations sounds like a contradiction, but all regions where bones
come together are called articulations, so there are articulations that don't
move, including in the skull, where bones have fused, and where your teeth meet
your jaw. Synarthroses are often joined with fibrous connective tissue.

Amphiarthrosis Articulations
There are some articulations which have limited motion called articulations, that are held in place with
cartilage or ligaments. Some amphiarthroses are permanent such as the
articulation between the ribs and the sternum (via costal cartilage).
Some are temporary such as the epiphyseal plate. Ribs connected to the
sternum (except the first rib) and the
two parallel bones in the arms and legs are
considered amphiarthrosis
articulations.

The articulations that people are most familiar with are
articulations, which have wide ranges of motion. Diarthrotic
articulations are said to be freely moveable and have a joint cavity. A
joint cavity is a structure that consists of a joint capsule which
surrounds the joint, and a synovial membrane which is inside the joint
and produces a fluid known as synovial fluid. These joints are known
as synovial joints and are further classified according to the type of
movement allowed at the joint.
Nonaxial Joints
Nonaxial joints do not have a pivot or axis of movement. An example are
gliding joints, also known as plane joints. These joints do not allow much
movement other than sliding and twisting. These are often found in certain
articulations in the wrist and ankle.
Gliding joint of the wrist

|
|
Uniaxial or Monoaxial Joints
These joints have one axis of movement and are more moveable than gliding
joints. Examples of uniaxial joints are hinge and pivot joints.
These joints have protrusions that fit into a corresponding depression; hinge
joints, such as the elbow, allow the movement of flexion and extension. The
pivot joint of the atlanto-axial joint (the atlas or C-1 on the axis or C-2)
rotation of the head and neck. For example, when you shake your head "no" you
are using this joint to rotate your head.
Elbow joint

|
|
Cross section of vertabra

|
|
Biaxial Joints
Biaxial joints have two axes of movement and therefore allow more
movements than uniaxial joints. One example of a biaxial joint are saddle
joints, such as the thumb. These joints were appropriately named because they
look like saddles; there are two curved surfaces that meet and allow limited
motion in many directions.
thumb joint

|
|
Another example of a biaxial joint is an ellipsoid or condlyar joint. Ellipsoid
articulations, also called condyloid articulations, have oddly shaped or
elliptical interactions that articulate to allow movement in two planes with no
rotation. These are present in the fingers and toes.
Finger joints

|
|
Triaxial Joints
As you can guess, triaxial joints are the most moveable with three axes of
movement. The ball and socket joints such as the shoulder and hip, have the
widest range of motion. They are aptly named as the head of the bone resembles a
"ball" and the articulating bone of the joint (either the scapula or coxal bone)
has a deep pit for the "socket".
Hip joint

|
|
The skeletal system is organized into the following structural hierarchy, from
microstructure to gross anatomy:
-
Chemistry and molecular scale - Water, minerals, collagen, and other proteins.
-
Nanoscale - Mineral crystals are embedded within collagen to form composite
fibers.
-
Submicron scale - Mineralized fibrils are organized into a (plural is "lamellae").
-
Micron (micrometer) scale - Lamellae form osteons in compact bone or trabeculae in
spongy bone.
-
Macroscale - Compact and spongy bone combine together to form whole bones, and bones
combine at articulations to form the skeletal system.
Bones constantly store and release calcium, phosphate, proteins, and other matrix
components of bone. Within the matrix, the mineral crystals resist compression but are
very brittle. The organic components, such as the collagen fibers and the cells, help
give the bone some flexibility. The hierarchical organization of the skeletal system at
different scales is important for its mechanical, biological, and chemical
functions.
The major components of bone tissue at the molecular scale are minerals, water,
collagen, and other proteins. At the next level of organization, small crystals of
hydroxyapatite made of calcium and phosphate are embedded within collagen fibers to
produce a composite (blended) material with high compressive and tensile strength.
The skeletal system protects and supports vital organs, allows our body to move,
stores important minerals, and produces blood cells. There are several chemical
elements and molecules required to maintain the many functions of the skeletal
system. The chemical properties of these components support bone structure and
function. On a chemical level, bone is divided into inorganic and organic
(carbon-containing) components.
The primary inorganic components of bone are:
-
calcium, which is required for many functions
throughout the body;
-
phosphorus (in the form of phosphate ions), which is a
component of buffer systems and energy-rich molecules; and
-
water, which contributes to the compressive resistance
of bone and contributes to the fluid matrix of bone.
The primary organic components of bone are:
-
collagen, the major structural protein (type I in bone
and type II in cartilage); and
- , which are negatively charged glycosylated proteins (glycosylated means
having carbohydrate sugar groups modifying the protein).
Bone is approximately 60 to 70 percent inorganic mineral and 10 percent water by
weight. The remaining 20 to 30 percent of bone is organic matrix (osteoid), such as
collagen and proteoglycans. Your body contains 1 to 2 kilograms of calcium and nearly
600 grams of phosphorous. Nearly 99 percent of the calcium and 86 percent of the
phosphorous is stored in your bones.
Calcium ions (Ca2+) are stored in bone tissue, but can be released into the
bloodstream when blood levels fall below optimal. Blood calcium is important for muscle
contractions, nerve impulses, and blood clotting.
In bone, phosphorous (P) is found in the form of phosphate ions
(H2PO4-). Outside of bone, phosphorous plays roles in energy
storage (such as in ATP), and is required for the formation of DNA and RNA. Therefore,
it is required for cellular growth, maintenance, and tissue repair.
When combined with hydrogen, phosphorous forms dihydrogen phosphate ions
(H2PO4-). Dihydrogen phosphate acts as a buffer to maintain a
constant pH balance by acting as either a hydrogen ion donor (acid) or a hydrogen ion
acceptor (base). In all cells, a constant pH must be maintained to carry out cell
functions.
As mentioned in previous sections, hydroxyapatite is an inorganic calcium phosphate
mineral that is a primary component of bone. Crystal hydroxyapatite has the chemical
formula: Ca10(PO4)6(OH)2.
During bone formation, the collagen fiber matrix is formed. Next, mineralization of the
matrix occurs when calcium, phosphate, and water from the extracellular fluids combine
to form insoluble hydroxyapatite. The hydroxyapatite incorporates into the small pores
within collagen fibrils and crystallizes into long, thin, nanosized plates within the
collagen network. The incorporation of hydroxyapatite within the collagen fibers
contributes to the overall compressive strength of bone. Because the crystals are
nanosized, they have a large surface area. The large surface area of the crystals makes
it easy to release ions into the extracellular fluids when blood levels decrease.
Sometimes bones contain fluoride. Fluoride is similar to the hydroxyl ion in charge and
size. When fluoride replaces the hydroxyl ion in hydroxyapatite crystals, it forms
fluoroapatite. Fluoroapatite is a more compact and less soluble crystal than
hydroxyapatite. Therefore, ion exchange at the crystal surface is slower. The primary
role of fluoride in bone is to strengthen bone mineral.
Water is an important molecular component of bone matrix. Water is initially present in
the spaces within the collagen matrix of the unmineralized bone extracellular matrix
(osteoid). As crystals fill in the spaces between collagen fibrils, the bone is
mineralized and the water is displaced. The water that remains in bone after this
process is complete forms hydrogen bonds with polar, hydrophilic components of collagen.
Water
also assists with the compressive resistance of bone due to osmotic interactions caused
by negatively charged proteoglycans.
In addition to calcium, phosphorous, water, and fluoride, the inorganic parts of bone
also contain sodium, potassium, carbonate, and magnesium. Although these molecules do
not form their own crystals, they are bound to hydroxyapatite crystals within bone
mineral.
You can think of the hydroxyapatite in bones as being similar to concrete. The
chemical structure of hydroxyapatite gives it a high mechanical strength under
compression (squeezing force), but little strength under tension (pulling force). If our
bones were made only of hydroxyapatite, they would be very fragile, like concrete. They
would break easily under tension. The organic components of bone help to give it
additional strength and flexibility.
Collagen, a ropelike, fibrous protein, is the major organic component of bone.
Like rope, collagen has significant mechanical strength under tension. Therefore,
collagen provides tensile strength to the bones. The spiral arrangement of collagen in
combination with its ECM (extracellular matrix) components, such as water and
specialized proteins, also makes bone more elastic and less brittle, so that it can
absorb shock more easily.
Bone matrix is a network of collagen fibers and hydroxyapatite. It is strong
under both tension and compression. Furthermore, the collagen contributes to its
ability to absorb sudden forces without breaking.
Collagens are a class of fibrous proteins found in bone, cartilage, and other
connective tissues. The primary structure of collagen has repeats of glycine-proline-X,
where X can be any other amino acid. This repeated arrangement gives collagen a unique
helical secondary structure. The
tertiary
structure of collagen is a triple helix fibril, which further twists into a quaternary
structure of a collagen fiber. The resulting "twisted, twisted,
twist" is similar to the higher-ordered structures in rope.
Type I collagen is the primary form of collagen found in bone. The collagen fibers in
bone are approximately 300 nanometers long, and are arranged in a staggered pattern.
During bone formation, the collagen fiber matrix is deposited first. Proteoglycans are a
second class of organic components found in bone.
Proteoglycans
are macromolecules composed of proteins with negatively charged glycosylated
modifications. There are a number of proteoglycans, which are
classified by the type of side chains on the core protein. The side chains on the
proteoglycans are linear carbohydrates. As with cartilage, the presence of the
negatively charged proteoglycans contributes to the resilience of the tissue and
retention of water within the matrix. Proteoglycans also bind and release molecules for
cell signaling.
Mineralization
of the bone matrix occurs when calcium, phosphate, and water from the extracellular
fluids combine to form insoluble hydroxyapatite. The hydroxyapatite incorporates into
the small pores within collagen fibrils and crystallizes into long, thin, nanosized
plates within the collagen network. The incorporation of hydroxyapatite within the
collagen fibers contributes to the overall compressive strength of bone.
Within the bone matrix, the minerals, water, collagens, and
proteoglycans function together to provide strength in tension and compression.
Additionally, this unique environment binds and releases chemicals into the
extracellular fluids for use throughout the body.
Example
Osteogenesis imperfecta (from osteo-, meaning bone, and genesis,
meaning production or beginning) is a congenital (genetic) disorder that affects the
structure and strength of bones. The condition is primarily caused by genetic
mutations that affect the structure and/or quantity of type I collagen produced by
the body. Remember that collagen is an important component of the extracellular
matrix of bone tissue. In people with osteogenesis imperfecta, the lack of collagen
(or presence of defective collagen) prevents the extracellular matrix from forming
correctly. The mineral structure of the bone is weakened, and as a result, the bones
are unusually brittle.
The bones of people with osteogenesis imperfecta break extremely easily,
sometimes with only small amounts of pressure. The bones' brittleness reduces their
ability to support and protect the body. They also typically take longer to heal and
may be more prone to infection.
Bones become stronger and thicker when they are exposed to regular stress.
Conversely, bones that do not experience regular stress may lose extracellular
matrix and become less dense. In a person with osteogenesis imperfecta, bones
that break often (and therefore spend a lot of time immobilized in casts or
restraints) are exposed to much less stress. As a result, they may lose even
more extracellular matrix and become even more brittle.
You have already learned about the hydroxyapatite and collagen combination, which
is responsible for giving bones their rigidity. The skeletal system is an excellent
example of a system that is primarily composed of extracellular matrix. Generally, cells
of the body function within a matrix to hold them together. In the case of bone, the
extracellular matrix (ECM) is composed of crystals of hydroxyapatite and long collagen
proteins. The hydroxyapatite and collagen work together to give bone its unique
properties of strength, compressive resistance, and flexibility.
Cells in Bone
The previous section described the importance of the bone matrix components
calcium and hydroxyapatite in maintaining bone rigidity. However, bone
remodeling is equally important to maintaining bone health. Throughout your
lifetime, your bones are constantly remodeled and repaired. There are three main
cell types in bone, which maintain bone balance and strength:
- , which break down and reabsorb bone;
- , which deposit and build new bone; and
- , which are mature bone cells that act as sensors for repair.
Think of the bones of your body as a rapidly expanding city. When the old
buildings get outdated, they are either remodeled to make enhancements or
knocked down to make room for the new ones. As soon as a building is knocked
down, a new one is erected in its place. The construction crew recycles the old
building materials. Many city planners constantly monitor the building progress
and talk with one another about which sections of the city will get the
available resources.
Osteoclasts secrete an acid that dissolves the inorganic components of bone:
calcium and phosphate. Osteoclasts also contain enzymes that digest the organic
components of the bone matrix, such as the proteins and proteoglycans. Bone
resorption (osteolysis) is complete when small cavities remain on the bone
surface. Osteoclast activity is important for creating the medullary cavities of
diaphyses, which house bone marrow. (Remember that the diaphysis is the main
shaft of a long bone.) Osteoclasts are important for removing calcium from bones
if the blood calcium levels fall, such as when a diet is deficient in calcium.
Remember calcium is critical for life and if levels fall, it could be fatal if
it wasn't taken from the bones.
Osteoblasts build bone and fill in the cavities created by osteoclasts.
Osteoblast cells secrete osteoid (the organic portion of the bone matrix) around
them, and then mineralize the matrix to generate rigid bone tissue. Bone
mineralization occurs through osteoblast secretion of an enzyme called alkaline
phosphatase. Alkaline phosphatase cleaves phosphate groups to provide the
necessary phosphate for building hydroxyapatite. Osteoblasts are stimulated when
blood calcium levels increase. The excess calcium is deposited into the bones
for storage.
After bone mineralization, osteoblasts trapped within the matrix differentiate
into osteocytes. Osteocytes have long processes
(outgrowths
or protrusions) for connection to other osteocytes within the
bone. This arrangement allows osteocytes to act as mechanical sensors.
Osteocytes sense mechanical strain within bone and regulate the activity of
osteoblasts or osteoclasts, depending on needs for calcium and phosphate. To
maintain bone strength, bone resorption and bone deposition must be in constant
balance with each other.
You have learned that bone tissue is classified into two types based on structure:
compact bone and spongy bone. The parallel arrays of lamellae are organized into
different arrangements depending on the bone structure. The lamellae in compact bone
form tubular structures, called osteons. The osteons of compact bone are oriented in the
direction of the load-bearing axis. The osteons also create a central canal for the
passage of blood vessels. Osteocytes in the osteons are embedded in small cavities
called lacunae
(singular
is ), and are oriented around the central canal parallel with the lamellae on the
load-bearing axis. The diaphyses of long bones are stronger on their long axis than in
any other direction, because of the parallel array of osteons and osteocytes.
The lamellae in spongy bone form a random mesh-like structure of interconnecting plates
called . Likewise, osteocytes within spongy bone are randomly arranged. The strongest
trabeculae in spongy bone are arranged on the bone axis that undergoes the most stress.
Flat bones of the
skull
are primarily made of spongy bone and are good at resisting forces from many directions
because of the trabecular arrangement.
In both types of bone tissue, the mineral components, calcium and phosphate, combine with
collagen to provide the compressive and tensile strength of bone. Spongy and compact
bone tissues are combined to create bones, which store and release calcium and phosphate
into the blood through constant resorption and deposition. Many bones then articulate
with each other to form the skeletal system.
Bones form in two ways. A process known as forms bones that develop from layers of connective tissue. Flat bones such as
those found in the skull develop through this process. Endochondral
ossification (from the word roots endo-, meaning
"within," and chondral, meaning "cartilage") is bone formation
from a hyaline cartilage blueprint or template, which determines the future bone shape.
Bones of the limbs and extremities develop through endochondral ossification. For
example, an infant's arm and leg bones contain only small amounts of actual hard bone
material; they are primarily made of cartilage. As the child grows, bone replaces the
cartilage.
Ossification is the process of forming bone. You learned that there are two types of
ossification:
-
intramembranous ossification, which is direct synthesis of bone by
specialized stem cells (mesenchymal cells) from fibrous connective tissue; and
-
endochondral ossification, which is synthesis of bone from a (hyaline)
cartilage template.
Intramembranous Ossification
Intramembranous ossification is the process that forms and repairs the flat bones
of the skull, clavicles and other irregularly shaped bones. In some situations
of bone repair and adaptation to excessive force, intramembranous ossification
generates new bone.
The process of intramembranous ossification involves multiple steps:
- Increased vascularization.
- Recruitment of mesenchymal stem cells
- Differentiation
- Secretion of osteoid
- Mineralization
- Formation of trabeculae
- Formation of outer compact bone
First, the site for future bone formation increases in vascularization—new blood
vessels form near the site where the bones will grow. Mesenchymal stem
cells, which originate in the embryonic mesoderm, become active and
travel through the blood vessels to the future site of bone formation. Chemical
messages then cause the mesenchymal stem cells to differentiate: they change
into osteoprogenitor cells, which may divide and differentiate into osteoblasts.
The osteoblasts deposit osteoid (the unmineralized bone extracellular matrix)
and are then trapped in the
matrix,
where they differentiate into osteocytes. Inorganic salts in
the blood travel through the blood vessels to mineralize the bone matrix. As a
result, hydroxyapatite crystals form within the osteoid. On the interior of the
tissue, small clusters of bone begin to connect with other clusters to form
trabeculae. Osteoblasts near the surface of the bone deposit matrix in organized
lamellae and form a thin outer layer of compact bone. The
periosteum ("peri-" means "surrounding" and "osteum" means
"bone") is living membrane composed of fibrous connective tissue that forms on
the outside of the compact bone. Its inside layer has osteoblasts for bone
growth and repair.
Endochondral Ossification
Most bones of the skeleton below the skull develop through endochondral
ossification.
This process involves the following steps:
- Formation of a cartilage template
- Growth of the template
- Differentiation
- Vascularization
- Calcification
- Bone formation
The first step is formation of a hyaline cartilage template, which is the shape
of the desired new bone. The cartilage template grows in size and thickens
through the production of new chondroblasts at the perichondrium. The
perichondrium is the cartilage equivalent of the periosteum. Chondroblasts
differentiate into chondrocytes, which produce chemical messages that stimulate
the increase of vascular supply at the perichondrium. This increase in vascular
supply brings in inorganic salts, which mineralize the central cartilage matrix.
Cartilage is laid down as a template that provides some mechanical stability.
This is like when designers and architects build a template out of balsa wood,
clay or foam because it is easy to quickly remodel and manipulate those
substances. Then, once the template is worked out, they will remodel it using a
stronger material. In bone, the 'model' cartilage is remodeled over time and
osteoblasts produce a full bone matrix with new collagen and hydroxyapatite. In
this way, biology works more efficiently than any engineered tissue graft.
After bone formation, bone resorption and bone deposition occur continually in a process called bone remodeling to allow for skeletal response to mechanical use, nutritional
status and as part of the bone repair and healing process. In the absence of
malnutrition or disease, this process maintains homeostasis of both total bone mass and
inorganic substances such as calcium and phosphate.
As bones age, they tend to decrease in density and, as a consequence, decrease
in strength. In some people, especially women, the bones become very brittle and
easily broken. The result is a disorder called . Within bones affected by osteoporosis, bone mass and
mineral content decrease. As a result, the bones develop canals filled with
fibrous and fatty tissues. This leads to an increased risk of bone fracture
because the bone organization that is important to weight bearing is lost.
The microscale structure of a bone gives it significant strength and rigidity,
but extreme forces can cause bones to break, or fracture.
The table below describes the most common types of fractures.
| Common Types of Fractures |
|---|
| Type of fracture |
Description |
|
Simple fracture (also called a closed
fracture) |
A fracture in which a bone breaks completely, but the broken
ends do not penetrate the skin |
|
Compound fracture (also called an open fracture) |
A fracture in which the ends of the broken bone break through
the skin |
|
Comminuted
fracture
|
A fracture in which the bone is shattered or broken into several
pieces |
|
Greenstick
fracture
|
A fracture that occurs in children when one side of a bone
breaks, and the other side bends |
|
Impacted
fracture
|
A fracture in which one end of the fractured bone is forced into
the interior of the other end |
| Stress fracture |
A set of tiny fractures or fissures in a bone caused by repeated
stress on the bone |
|
A bone fracture affects the bone on several levels of organization. A fracture
involves a physical break in the mineral structure of the bone. Fractures
typically cause blood vessels in the bone to rupture, reducing the blood flow to
the bone tissue. As a result, some of the cells in the surrounding bone die.
These dead cells and the related cellular debris are removed by immune cells and
osteoclasts. Over time, various cell types—fibroblasts, chondroblasts, and
osteoblasts—work together to repair the mineralized bone tissue.
Fractures weaken bone, making it less able to perform its functions of
support and protection, although once healed, the site of the fracture is stronger than
the remaining bone. A fracture may cause a change in the shape of the bone. If that
happens, then the way the bone responds to a contracted muscle may change. As a result,
fractures can prevent bones from moving correctly when muscles pull on them.
There are pain receptors and nerves in the bone. The pain experienced when a
fractured bone moves is one way the body reacts to help itself heal. Bones heal
more quickly and thoroughly if they are kept immobilized (which is why the
typical treatment for a broken bone is to put it in a cast or other restraint).
Because we instinctively avoid actions that cause pain, the pain that occurs
when a broken bone is moved causes us to minimize the movement of that bone.
This, in turn, helps keep the bone stable while it heals.
Severe fractures, such as compound fractures or comminuted fractures, can cause
long-term or permanent disruptions to the body's homeostasis. Because a compound
fracture breaks through the skin, bacteria and other pathogens can enter the
body after a compound fracture. Those pathogens can enter the bone, blood,
muscles, or other tissues or organs, causing severe infection. Severe fractures
are also less likely to heal correctly without medical (typically surgical)
intervention. Improperly healed fractures can cause changes in the bone's
reaction to force, leading to changes in body motion (such as a limp).
The skeletal system provides an excellent example of homeostasis. From the time that we
are born, our skeleton must be sufficiently robust to provide stature, protect our soft
internal organs and provide a mechanical resistance for our muscles to pull on. However,
in addition to the constant need for the skeleton to maintain its function, it must also
constantly remodel, otherwise we could not grow. Even in adulthood we constantly develop
microfractures that need to be repaired.
The body uses the bones not only for structure and protection, but also for calcium
storage. Approximately 99 percent of the body's calcium is stored in the bone, and
calcium plays an important role in most of the body's functions. Free calcium levels
must remain at a set
point for proper body functions. The extracellular levels of calcium
are affected by calcium intake from foods, excretion of calcium as waste, and the
storage and release of calcium from the bones. Hormones regulate these processes to
maintain balanced calcium levels in the extracellular fluid, which is necessary to
maintain homeostasis.
Small changes in blood calcium levels can have significant effects on body function.
For example, if extracellular calcium levels are too low, the nervous system becomes
overexcited, resulting in tetany (rigid, locked muscles). On the other hand, if calcium
levels in the body are above normal, the nervous system becomes sluggish. Muscle
activity of the heart and gastrointestinal tract slows down.
Milk and dark green vegetables are rich in calcium, and an adequate supply of calcium
helps maintain healthy bones. When we eat calcium-rich foods, some calcium is absorbed
into the small intestinal wall, and some of the calcium becomes soluble in the blood
stream. However, calcium and other divalent
cations
(ions that are missing two electrons) are poorly absorbed by the
small intestine. For this reason, vitamin D is an important dietary supplement. Vitamin
D increases calcium absorption in the small intestine.
By altering the function of the osteoblasts and osteoclasts, hormones help regulate
calcium levels in the blood. There are three hormones that control osteoblast and
osteoclast activity:
-
parathyroid hormone or PTH, which increases bone resorption by
stimulating osteoblasts, leading to increased calcium release from bone.
-
calcitonin, which acts in children to decrease bone resorption,
leading to less calcium entering the blood.
-
calcitriol, which increases absorption of dietary calcium.
- calcitonin, which acts to decrease bone resorption and increase bone deposition by stimulating osteoblasts.

Parathyroid hormone (produced in the parathyroid glands) controls extracellular
calcium by regulating how calcium is reabsorbed in the intestines, excreted from the
body, and exchanged between the extracellular fluid and the bone. The cells of the
parathyroid gland synthesize and release parathyroid hormone in response to low blood
calcium levels. To bring blood calcium levels into the normal range, the release of
parathyroid hormone stimulates osteoclasts to reabsorb bone mineral, therefore releasing
calcium into the blood. Parathyroid hormone also enhances calcium absorption by the
intestines, and prevents calcium loss in urine to increase calcium levels in the blood.
When parathyroid cells sense that blood calcium levels are above normal, cellular
receptors are activated and the synthesis and release of parathyroid hormone is
inhibited.
Calcitonin is produced in the thyroid gland. The function of this hormone is to
decrease bone resorption and retain calcium in the bones. Therefore, the effects of
calcitonin counteract the effects of parathyroid hormone. When blood calcium levels are
high, the thyroid releases calcitonin into the blood. Calcitonin decreases bone
resorption by decreasing the activity of osteoclasts and decreasing the formation of new
osteoclasts. In this way, calcitonin shifts the bone balance in favor of bone
deposition, which requires the removal of calcium from the blood and into the bone.
Calcitonin also has minor effects on how the intestines and kidney tubules handle
calcium. In adult humans, calcitonin has weak effects on the regulation of calcium
levels. We know this because if the thyroid gland is removed, calcium levels are not
adversely affected.
Vitamin D is a group of lipid soluble compounds involved in
calcium regulation. Vitamin D can be made in the skin through exposure to sunlight, or
acquired from the diet and dietary supplements. Under the influence of the parathyroid
hormone, vitamin D is converted to an active molecule, calcitriol.
Calcitriol circulates through the blood to maintain normal blood calcium levels. When
your body does not have sufficient levels of calcitriol, the intestines do not absorb as
much calcium, and blood calcium levels decrease. Vitamin D also inhibits calcium loss in
urine.
The activity of osteoblasts and osteoclasts in the bone is tightly regulated by the
activity of the parathyroid hormone, calcitonin and vitamin D. Nutritional deficiencies
in vitamin D, calcium or phosphate lead to a disease called osteomalacia.
Osteomalacia (from osteo-, meaning "bone," and mal-, meaning "bad"), also known as rickets when it occurs in
children, is a disease that is most commonly caused by a lack of vitamin D in the body.
Remember that vitamin D helps to increase the amount of calcium that the body absorbs
and, as a result, helps the body build the mineralized extracellular matrix in bone
tissue. If a person lacks sufficient vitamin D, calcium absorption is slowed, and bone
growth is affected.
In children, the lack of calcium absorption prevents new bone tissue from ossifying
properly. As a result, the bones become weak and rubbery. Because their bones are less
able to support the body and withstand the pressure of the body's weight, children with
rickets may develop bowed legs and deformed skeletons.
In adults, osteomalacia prevents bones from healing and remodeling properly.
Recall that bone tissue is being continually formed and broken down by
osteoblasts and osteoclasts. If insufficient calcium is available, the new bone
that forms may not calcify properly. As a result, the bones may become painful
and brittle.
Osteoporosis is a condition in which the balance between
calcium deposition and calcium loss in bones is disrupted. In people with
osteoporosis, the bones lose more calcium than they deposit. As a result, the
bones become brittle and break easily.
Osteoporosis is most common in post-menopausal women. As a woman goes through
menopause, her levels of estrogen decrease significantly. Estrogen and testosterone are
important hormones that affect the reproductive system, but they also can stimulate
osteoblast activity. As concentrations of these hormones decrease, osteoblasts become
less active, and less calcium is deposited in the extracellular matrix. In addition to
resulting in weak bones, this is also an issue because there is a reduced reserve of
calcium in the body for other tissues.
Osteoporosis can be affected by diet and lifestyle. People who consume very
little calcium and vitamin D are at increased risk of osteoporosis. (For
example, women with anorexia nervosa, who eat very little, may develop
osteoporosis in their late twenties and thirties.) Weight-bearing exercise, such
as running, helps to build bone mass and can reduce the chances of developing
osteoporosis.
You learned that osteocytes become embedded within bone matrix during mineralization.
Within bone lamellae, osteocytes interact with each other through a network of cellular
extensions. These extensions, found in a network of canals referred to as canaliculi, allow external mechanical information to be rapidly
transmitted to many osteocytes. The response of osteocytes to mechanical force allows
our skeletal system to maintain itself.
When bone experiences a mechanical force, this force is transmitted to the fluids and
cells within the bone. The forces on the fluids are then transferred through the spaces
of the canaliculi down to the osteocytes. In response to the received forces, osteocytes
generate a force on the bone tissue. Therefore, the cells and bone matrix constantly
respond to each other’s mechanical force input.
Osteocytes in bone tissue act as mechanical strain sensors to convert information
about mechanical force into chemical messages. These chemical messages control
osteoblast and osteoclast activity and can result in either bone deposition or bone
resorption, also known as osteolysis. In response to mechanical force, a two-step
process occurs. First, mechanically sensitive membrane proteins on the surface of the
osteocytes are activated. Second, chemical pathways within the osteocytes are activated.
Activation of these intracellular pathways stimulates osteoblast activity and inhibits
osteoclast activity.
As you learned earlier, bone ECM (extracellular matrix) maintains the mechanical
structure and functional properties of bone tissue. The collagen fibers of bone ECM are
under constant tension so that the bone ECM is always ready to respond to mechanical
force. Because osteocytes are attached to the ECM, the tension felt by the collagen
fibers is balanced by the tension that osteocytes place on the ECM. Therefore,
osteocytes sense mechanical force and transmit this force to the surrounding ECM. Bone
ECM is constantly remodeled to maintain optimal strength for applied mechanical
force.
Mechanical forces control osteoblast and osteoclast activity through feedback from
the osteocytes and the ECM. One common type of mechanical force is physical activity.
Another force that is not as obvious is body mass. With increased mechanical force, such
as physical activity or weight gain, bone ECM increases to support the force. When the
mechanical force applied to bone is too high, osteocytes within bone matrix signal for
remodeling through bone resorption and bone deposition.
People who routinely lift heavy loads have very strong bones, but the body spends a
lot of energy to maintain these stronger bones. On the other hand, sedentary individuals
may lose bone density and strength. However, that can be a problem: a lack of exercise,
extended bed rest and space travel (for astronauts), during which there is little or no
force on bones because of inactivity or weightlessness, can cause a significant loss of
bone mass.
You have learned in relation to ossification how cartilage and bone are
inherently integrated. Both tissue types are also some of the most
force-responsive in the body. This is necessary to maintain stature.
Chondrocytes sense force and changes in water movement—associated with the
force-induced movement of water—and secrete the appropriate collagen and
proteoglycan extracellular matrix proteins.
Recall that cartilage is composed primarily of a network of elastic
type II collagen fibers embedded in gel-like
proteoglycans. Water, electrolytes, and chondrocytes are interspersed
within the network. The physical properties of collagen and proteoglycans are
largely responsible for the ability of cartilage to respond flexibly to
force.
Collagen fibers have significant tensile strength, which
means that they can withstand a lot of tension (pulling) without damage.
However, collagen fibers have very little compressive
strength—that is, under compression (squeezing), they bend easily. You
can think of a collagen fiber as a rubber band or string. If you pull on a
rubber band, it stretches easily, and then returns to its original shape when
you stop pulling. However, if you push on the ends of the rubber band, it folds
up easily—it has minimal strength.
Collagen fibers are what give cartilage its strength. The cartilage in different
areas of the body contains fibers that are arranged in different ways. The
orientation and arrangement of the collagen fibers helps to give each region of
cartilage strength to withstand specific types of stress. For example, articular cartilage—the cartilage that forms the
articulating surfaces of joints, such as the knee—contains two main regions of
collagen fiber orientation. At the surface of the cartilage, the fibers are
arranged primarily parallel to the surface. Further from the surface, the fibers
are arranged primarily perpendicular to the surface.
Proteoglycans are gel-like, elastic substances. They are highly resilient, which
means that they deform easily under stress, but return to their original shape
when the stress is removed. Proteoglycans give cartilage its resiliency and
elasticity.
The combination of strength and elasticity allows cartilage to respond flexibly
and quickly to forces placed on it. For example, the cartilage in your knee can
support up to eight to 10 times your body weight for short periods without being
damaged. As more force acts on the cartilage, it compresses. Its elasticity
allows it to distribute the force evenly, and its strength allows it to
withstand the stress without breaking or collapsing. When the stress is removed,
the cartilage returns to its original shape. The physical movement of the water
in the cartilage also helps to distribute the force of compression.
Cartilage is less complex in response to force than bone, which has multiple
integrated cell types. Cartilage is less regulated by hormones, is difficult to
overgrow, and grows extremely slowly. Part of the reason for slow growth is a
limited amount of nutrients. Cartilage is avascular (a- means "none"), so there
are no blood vessels or blood supply to
the
interior of the cartilage, so nutrient travel is limited to
diffusion, and growth is limited.
Arthritis (from arthro-, meaning "joint," and –itis, meaning "inflammation") is any inflammation of the
cartilage and/or bone tissue within a joint. The most common form of arthritis
is osteoarthritis, which is caused primarily by physical
damage to the cartilage that cushions many joints. The damage may be caused by a
decrease in cartilage flexibility and water content as a person ages, but it may
also be a secondary result of a variety of other conditions (including diabetes
and mechanical injury to the joint). Osteoarthritis is most common in the
elderly, but can affect younger people as well.

Osteoarthritis begins with physical damage to cartilage tissue in the joints. The
damage may be cellular (such as damage cause by an infection), or it may occur
at the tissue level. The damage causes the cartilage to break down. As the
cartilage breaks down, friction within the joint increases; in severe cases, the
ends of the bones themselves may rub together, causing severe pain. Loss of
cartilage can also reduce the flexibility of the joint. Pain and loss of
flexibility typically cause a person to move the joint less; as a result, the
muscles, tendons, and ligaments near the joint may weaken. This in turn can
cause additional difficulty moving the joint.
A different form of arthritis that also causes joint pain,
rheumatoid arthritis, is caused by inflammation of the membranes of the
synovial capsule, not the articular cartilage.
The homeostatic control of blood (plasma) calcium levels requires interactions between
several body systems. Roughly 1000 mg of calcium per day is taken into the body by
ingestion, 350 mg of calcium per day is absorbed into the digestive system
and the rest is excreted. Of the calcium absorbed by the digestive system and from there
into the cardiovascular system, some is processed through the urinary
system. Calcium exchange between cells, extracellular fluid and the bones is
partly regulated by the endocrine system, which directs bone degradation if
more calcium is needed in other parts of the body. The endocrine system
also stimulates osteoclasts, which are cells derived from the precursor cells of the
immune system. The calcium liberated by the bones is used by the nervous
system and the muscular system to keep many of their functions
working appropriately.
Systems within the body are integrated with respect to dysfunction as well. Osteoarthritis is
the most common form of arthritis, and as you have learned, it is caused by mechanical
damage to the cartilage. Rheumatoid arthritis, in contrast, is an
autoimmune disease—that is, a disease in which the body's
immune system attacks normal tissue. In rheumatoid arthritis, the immune system attacks
healthy cartilage and synovial membranes.

Immune cells attack the synovial membranes in synovial joints, causing inflammation. As the membrane
becomes inflamed and thickened, synovial fluid begins to accumulate in the joints. The joints can
become swollen and painful, and it becomes more difficult for the bones in the joint to slide past
one another. Over time, fibrous tissue forms in the joint. Eventually, the fibrous tissue fuses the
two bones together, preventing the joint from moving.
When you think of muscles you probably have a picture in your mind of some muscular
people who are pleasing to the eye. Did you know that you have over 650 muscles in your
body? That sounds like a lot but a caterpillar has even more, around 4000 muscles!
Without muscles we would never be able to move. The main function of the muscular system
is movement. But did you know that muscle contractions generate 85% of our body heat?
Muscles also protect our organs. Your abdominal muscles keep your “guts” and internal
organs in place. Some muscles are circular, such as the one that encircles your mouth
for kissing or puckering. Other circular muscles form sphincters which control when you
defecate or urinate. One important muscle, known as your diaphragm, is necessary for
breathing. If your diaphragm stopped working you
would die in minutes because you would not breathe.
Let’s test your knowledge on some common facts and myths of the muscular system.
Let’s check out the muscular system in action in the following case study.
Seymour is a nineteen year old power lifter and college student. You sit next to Seymour
in psychology class and have gotten to know him this semester. On Monday, he sits down
next to you and lets out a wince of pain as he takes his seat. You ask him, “What’s
wrong?”
He replies, “Oh, it’s probably nothing, but my stomach has been hurting especially when I
lift something heavy.”
“Don’t you work at The Dark Horse Tavern? And work out a lot? I bet that must be tough on
you. Do you think you pulled a muscle?” you ask him.
Seymour answers, “I don’t know, I’ve never felt this kind of pain before, it hurt a lot
at work and I haven’t been able to lift as much at the gym.”
“Why don’t we check this out on my iPhone, we still have 5 minutes before class begins”
you reply.
Seymour suggests going to WebMD.com for a quick look. “Hmm, I don’t see anything specific
for stomach pain while exercising,” you tell him. “What other symptoms do you have?”
“Well, it’s kind of embarrassing but I have noticed this lump in my navel especially
while lifting.”
“My cousin had something like that before, I think it was called a hernia, let’s Google
that. Oh – man, it sounds like something called an umbilical hernia, it says that the
bulge might be your intestines!” you whisper to him. “Seymour, you better get to your
doctor right away, this can’t be good.”
Seymour goes a little pale and says, “I’m going to call my mom right after class.”
The muscular system is mainly composed of skeletal muscle, such as those that cover the
anterior portion of your abdomen and help compress the abdominopelvic cavity and
digestive organs. As we saw with Seymour, if these muscles are weakened, they might
separate and allow the underlying organs to protrude. Seymour’s constant exposure to
high intra-abdominal pressure has weakened the muscle. If a piece of Seymour’s intestine
is bulging through his abdominal muscles this can have serious consequences. The small
intestine is a critical component of the digestive system and if it is pinched off that
section could become necrotic and die.
There are two other types of muscle tissue that are necessary for
life: cardiac and
smooth muscle. Cardiac muscle is found in the heart and its rhythmic
contraction is responsible for your heart beat, while smooth muscle is
found in many organs and blood
vessels. Smooth muscle is a significant part of your cardiovascular
system (blood
vessels), respiratory system (bronchioles), digestive system
(esophagus, stomach, small
and large intestines), urinary system (ureters and urinary bladder),
and your
reproductive system (uterus, vas deferens).
As stated earlier, skeletal muscle has many important functions in maintaining our
homeostasis. Skeletal muscle contractions are critical in producing heat for our body.
In fact, when you are very cold your skeletal muscles increase contractions to generate
more heat to warm you – this is known as shivering. When you exercise and your skeletal
muscles are more active you produce more body heat and your body temperature increases.
Your body will sweat to help cool itself.
The diaphragm is a skeletal muscle that contracts to allow us to inhale, we exhale when
it relaxes. Any disruption in this important muscle’s function can be fatal. The
diaphragm is a critical organ of the respiratory system.
Clearly, skeletal muscle and muscle tissue is much more important than just giving us a
“buff” body!
The muscular system consists of skeletal muscle connected to bones via tendons. Tendons
are a type of dense regular connective tissue that joins muscles to bone. The abdominal
muscles are also held with broad tendon sheaths termed aponeuroses. In Seymour’s case
either the muscle separated or pulled away from its aponeurosis that helped to anchor
the muscle fibers together.
As mentioned, there are three types of muscle tissue. Skeletal muscle attaches to bones,
is voluntary, and has a striped (striated) appearance. Cardiac muscle is found in the
heart, is involuntary and is striated. Smooth, sometimes known as visceral muscle, is
found in many organs and blood vessels, is not striated but is involuntary.
All muscle tissue is composed of muscle cells known as muscle fibers. These fibers are
bundled and held together with connective tissue. The basic unit of the muscle cell is
the sarcomere. Muscles contract because sarcomeres shorten. Calcium is essential for all
types of muscle tissue to function properly. You will investigate the role of calcium in
muscle contraction in this module.
Muscle contains many proteins, mainly myosin and actin. Skeletal muscle is under control
of the nervous system and will not contract unless a neural command reaches the muscle
and instructs it to do so. The nervous system communicates with skeletal muscle via
chemical messengers known as neurotransmitters. The neurotransmitter, acetylcholine, is
responsible to stimulating skeletal muscle so it contracts. The process of muscle
contraction occurs at the cellular level and will be studied in this unit.
Muscle fibers or cells are not all alike. There are cells that are specialized for
endurance events, these are known as slow-twitch fibers. There are also cells or fibers
that are better suited for power and sprinting. These fatigue a lot faster and are known
as fast-twitch fibers.
When you study the muscular system you will need to understand the microscopic anatomy
and physiology so you can learn how muscle contracts. You will also study the gross
anatomy of muscles, their names, locations, and functions in the human body. The
muscular system is a fascinating system. Enjoy your journey as you discover it!
Skeletal muscles (organs) are almost never completely relaxed. Even if a muscle is not
producing movement, some of the muscle fibers (cells) in the organ contract to
produce muscle tone . The tension produced by muscle tone prevents the muscle from becoming
weak, allowing the muscle to stabilize joints and maintain posture. Muscle tone
is accomplished by the contraction of a small fraction of muscle cells so
muscles won't fatigue completely; some cells can recover while others are
active. This can help with stronger movements, as the motor units being used to
maintain tone can produce a quicker, stronger contraction than the motor units
that are resting, avoiding the that occurs with muscle fibers that are stimulated
from a state of rest.
The absence of the low-level contractions that lead to muscle tone is referred to as
hypotonia. Hypotonia can be caused by nervous system dysfunction or imbalances
in certain ions in the blood stream. Hypotonic muscles have a flaccid appearance
and display functional impairments. Conversely, excessive muscle tone is
referred to as hypertonia. This takes two forms: spasticity, which is a type of
stiffness related to uncontrolled reflexes, and rigidity, a stiffness not
associated with reflexes.
Isometric and Isotonic
There are two main types of skeletal muscle contractions: isotonic and isometric. When a
muscle exerts a force, it exerts this force on a load. At the molecular level,
cross-bridge formation occurs in both isotonic and isometric contractions, but
the whole muscle acts differently depending on the load and the outcome to be
achieved. In isotonic (iso- same, tonic - tension)
contractions, a load is moved as the length of the muscle changes.

There are two types of isotonic contraction: concentric and eccentric. Concentric contractions involve the muscle shortening to move a load.
An example of this is the biceps muscle shortening as a hand weight is brought
upward and the angle of the elbow decreases. Eccentric
contractions occur as the muscle lengthens. Eccentric contractions do not
produce adequate force to move a load but are used for movement, balance, and
resisting movement. An example of an eccentric contraction is the movement of a
bicep as it performs the lowering portion of a biceps curl. A concentric
contraction brings the arm upward, reducing the angle of the elbow, and then an
eccentric contraction lowers the arm, resisting gravity and increasing the elbow
angle slowly. The same muscle tension is required whether the muscle is
shortening or lengthening.
Isometric (iso- same, metric- length) contractions occur as the muscle
produces tension without changing length. Isometric contractions do not produce
movement or lift loads; these contractions maintain posture and joint stability.
For example, pushing against a wall produces no movement because the muscle
simply cannot produce enough force and thus does not result in changes in muscle
length. Conversely, holding your head in an upright position occurs not because
the muscles cannot move the head, but because the goal is to remain stationary
and not produce movement. In both of these cases, cross-bridge cycling occurs in
the same manner as it would if the load had been moved. Most actions are the
result of a combination of isotonic and isometric contractions working together
to produce a wide range of outcomes.

All of the contraction that occurs before a load is lifted is isometric. Even when muscles
carry no load, they must still develop a tension equal to the muscles' own
weight before they can shorten. When contractile forces exceed the weight of the
load, muscles begin shortening. Shortening stops when active tension falls to
the point that it can no longer overcome and move the load. This converts it
back to an isometric contraction.
learn by doing
It is relatively easy for someone to lift a glass of water. However, holding a glass of
water at arm’s length for one full minute is difficult.
Skeletal muscles, which are the organs of the skeletal muscle system, include three layers of
connective tissue that enclose and provide structure to the muscle as a whole. The outer
layer of each muscle fiber is wrapped with a layer of connective tissue called the
epimysium ("epi-" means "outside" or "over," and "-mysium" refers to
"muscle"). The epimysium allows muscle to contract and move powerfully while maintaining
its structural integrity; it also separates muscle from other tissues and organs. It is
composed of a thick layer of collagen fibers. The epimysium is surrounded by
fascia, which is a type of connective tissue that is found around body
organs. Deep fascia surrounds groups of muscles, sometimes joining with tendons to
strengthen the bone attachment, whereas superficial fascia lies between muscle and skin.
Some fascia contain adipose tissue that insulates and protects muscle.
Inside each skeletal muscle, muscle fibers (a single muscle cell is called a muscle fiber; not
to be confused with fibers found in connective tissue) are organized into groups called
fascicles, or bundles of muscle fibers (cells). Some of these fascicles can be seen
without a microscope when a muscle is cut open. Each fascicle contains many long muscle
fibers bound by a middle layer of connective tissue called the perimysium
("peri-" means "around"). Similar to the epimysium, the perimysium contains collagen
fibers, but the perimysium also contains elastic fibers.
Inside each fascicle, each individual muscle fiber is encased in a connective tissue
layer called the endomysium ("endo-" means "inside"). The
endomysium forms a thinner layer than the dense epimysium and perimysium, and contains
areolar and reticular tissues which form loose, delicate networks. The endomysium of
muscle fibers connect to each other to form a loose complex within a fascicle. Small
blood vessels and motor neurons pass through the endomysium to support and activate each
muscle fiber.
The plasma membrane, or sarcolemma, of a skeletal muscle fiber is located just under the
endomysium. The sarcolemma is the site of action potential conduction, which triggers
muscle contraction. Within the sarcolemma is the sarcoplasm, the cytoplasm of the muscle
cell.

Example
Muscular Dystrophy (MD) is a progressive weakening of skeletal
muscles. Duchenne’s Muscular
Dystrophy (DMD), the most common type of MD, is caused by a mutation
in the dystrophin gene. Dystrophin helps the thin filaments of
myofibrils bind to the sarcolemma
and maintains equal force transmission through the muscle tissue.
Without sufficient
dystrophin, muscle contractions cause the sarcolemma to tear,
causing an influx of
Ca2+, leading to cellular damage and muscle fiber degradation. Over
time, muscles are damaged and functional impairments develop.
DMD is an inherited disorder caused by an abnormal gene found on the X chromosome. It is one
of many genetic diseases which are referred to as X-linked; X-linked disorders
affect males since they only carry one copy of the X chromosome, which they
inherited from their mother. Girls inherit one X chromosome from their mother and
one from their father, so they are usually not affected. DMD is usually diagnosed in
early childhood. It usually first appears as difficulty with balance and motion, and
then progresses to an inability to walk. It continues progressing upwards in the
body from the lower extremities to the upper body, where it affects the muscles
responsible for breathing. It ultimately causes death due to respiratory failure,
and those afflicted do not usually live past their twenties.
The fascicles of parallel muscles are arranged parallel to the long axis of the muscle.
They are equidistant and run in the same direction. The majority of skeletal muscles in
the body follow this type of organization. With muscles that seem to be plump, the large
mass of tissue located in the middle of the muscle is known as the central body, but its
more common name is the belly. When the muscle contracts, the contractile fibers shorten
into a large bulge. To see this, extend and then flex your biceps muscle. The large mass
of the muscle is called the belly; tendons emerge from both ends of the muscle. When a
muscle contracts, it is the pulling on the tendon by the muscle that actually produces
the movement.
The fascicles of circular muscles, also called sphincters, are arranged around an opening in
rings. When the muscle contracts, the size of the sphincter opening decreases. The
orbicularis oris muscle is a circular muscle around the mouth under the lips. Another
example is the orbicularis oculi ("ocular" means "eye"), which surrounds each eye. The
circular muscles expand and contract in a circular way. The circular muscles contract
and relax in a circular way. Think about how you can contract and shape your lips to
whistle or pucker.
In convergent muscle, the fascicles extend from a broad, fan-shape area and converge on single
attachment site where the muscle interacts with a tendon. .The large muscle on the
chest, the pectoralis major, is an example of a convergent muscle. Because of the
arrangement of motor units within a convergent muscle, it can be stimulated in
different, smaller areas. This can change the direction of the muscle force slightly
from when the entire muscle contracts at once.
Pennate muscles are feather-shaped and form different fascicle arrangements at an angle
to the tendon. Contracting pennate muscles pull at an angle and can produce high
tension, but don’t move tendons very far. There are three subtypes of pennate muscles:
unipennate, bipennate, and multipennate muscles. In unipennate muscles, such as the
extensor digitorum in the forearm, the fascicles are located on one side of the tendon.
A bipennate muscle, such as the rectus femoris in the thigh, has fascicles on both sides
of the tendon. If the tendon branches within a pennate muscle, as it does in the deltoid
muscle of the shoulder, the muscle is referred to as multipennate.
Some parallel muscles are fusiform and have fascicles that are
spindle-shaped to give the muscle a large belly, such as the biceps brachii, whereas
triangular muscles can be convergent or multipennate to create triangular shapes. Triangular muscles include the trapezius, which extends
from the head down the back and out to the shoulder.
Skeletal muscles, which are the organs of the skeletal muscle system, generally act by
pulling on bones to produce movement. To pull the bone, muscles need to attach to the
bone, either directly or indirectly. The epimysium of a muscle can attach directly to
bone or cartilage to form a direct attachment. An indirect attachment is formed when the
connective tissue layers, the epimysium, perimysium, and endomysium form a complex at
the end of the muscle.
For connections, muscles require either a tendon or a broader sheet of connective tissue called
an aponeurosis. Muscles connect to muscles via aponeuroses, and muscles attach to bones
via tendons or aponeuroses. Thus, when a muscle contracts, the force of movement is
transmitted through the attachment, which pulls on the bone to produce skeletal
movement.
Tendons also help to stabilize the joints. Tendons are a common form of attachment
because the collagen fibers are more resistant to tearing than direct muscle tissue
attachment to bone would be, and the compact form of the tendon requires a small amount
of space. Tendons can be easily seen as the hand is flexed, causing the thick, cordlike
tendons of the forearm to stand out prominently. The calcaneal (Achilles) tendon is
visible from the heel to the calf. Some tendons are also surrounded by a connective
tissue layer called a tendon sheath, which protects the tendon as it moves. Fluid-filled
sacs called bursae also join to tendons to reduce friction as the tendon moves. Bursae
are present in connective tissue near bone, where tendons experience friction, but they
occasionally arise in other areas due to stress caused by movement.
Example
Bursae can become inflamed, a condition known as bursitis. This is usually caused by overuse
of a joint or by other mechanical stress, which can result in pain and swelling.
Bursitis is common in knees, elbows, and shoulders.
Keep in mind that the range of motion produced by muscles is restricted by the anatomy of
the bones and other support structures involved in a particular joint. Consider the
movements of the hip and shoulder joints, both of which are freely moveable. The hip is
the attachment point for the thigh and leg. The shoulder is the attachment point for the
arm and forearm. The shoulder can produce movements that the hip cannot because of the
anatomy of the bones involved and the ligaments (connective tissue) associated with the
joint. Hold your arm out to the side and move your thumb around the axis of the arm in a
360-degree circle. Can you do that with your hip and leg? No, you can’t. There are some
people who appear to be
“double-jointed";
they are able to push their joints past the normal limits of movement. The reason they
can do this is because the ligaments of their joints are “loose” compared with someone
who is not “double-jointed.” This ability appears to have a genetic basis.
For muscles attached to the bones of the skeleton, the location of the connection
determines the force, speed, and type of movement. These characteristics depend on each
other and can explain the general organization of the muscular and skeletal systems.
We can consider the mechanisms by which muscles act on bones using descriptions based on
levers. A lever is a rigid structure, in this case a bone, that moves on a fixed point
called the fulcrum, in this case an articulation. A lever moves when an applied force or
effort is sufficient to overcome any load or resistance that would otherwise oppose or
prevent such movement. In the body, each bone is a lever and each joint is a fulcrum,
and muscles supply the applied forces. Movement of the skeleton occurs at joints, so
there has to be sufficient muscle power to move all the bones at these joints.
Levers can change the direction and effective strength of a force as well as the distance and
speed of movement produced by the force. Imagine a seesaw in a playground. You and a
friend could sit on opposite ends of it. You would then take turns pushing off the
ground with your legs as you each went up in the air. A seesaw is an example of a
first-class lever, and there are three classes of levers. The fulcrum (F) lies at the
midpoint of the seesaw, between the applied force (AF) and the load (L). What this means
is that the seesaw balances you and your friend, as you both provide the applied force
with the push of your legs and the load with the weight of your bodies.


The body has only a few first-class levers. One is involved in head
flexion (pulling the head down toward the chest) and head extension (pulling the head
back up into normal position). The fulcrum is where the head moves in the sagittal plane
on the first cervical vertebrae. The load is the weight of the head, and the applied
force comes from the muscles. Extension occurs when the muscles pull the head back up.
Moving your head forward and backward mirrors the action of a seesaw.
In a second-class
lever, the load is located between the applied force and the fulcrum.
When you move a load in a wheelbarrow, you lift upward on the handle and the wheel acts
as the fulcrum. The force is further from the fulcrum than the load is, so you can move
a larger weight with less effort. In the body, you achieve the same effect by standing
on your toes. The fulcrum is on the ball of the foot, the load is your body weight, and
the effort comes from the muscles in the back of the leg.
In third-class
levers the force is applied between the load and the fulcrum. We don’t
often see these types of levers in artificial machines, but they are the most common
levers in the body. Speed and distance traveled are increased at the expense of force.
For the biceps brachii in the arm, the load will be located in or around the hand. The
biceps muscle applies the force, while the fulcrum is the elbow. For instance, when you
pick up a full bag of groceries, you can lift it quickly using the third-class lever of
the biceps brachii and the forearm. However, the location of fulcrum prevents great
enhancements of load carrying.
Skeletal muscles are arranged in pairs for balance and to work efficiently.
Some muscles function as agonists, or prime movers. An antagonist is a muscle that
opposes the action of the agonist. When an agonist contracts to produce a movement, the
corresponding antagonist will be stretched and contract with sufficient tension to
control the movement. For instance, when your biceps muscle acts to flex your forearm,
the triceps muscle contracts slightly to prevent you from flexing your forearm too
quickly or too strongly.
Agonist and antagonist muscles work against one another to control and balance movements.
Other muscles, called synergists (from synergy, to work together), improve the
efficiency of an agonist muscle. Synergists may provide additional pull or
stabilization, and their assistance to a particular movement may change throughout the
progression of the movement.
This table provides examples of agonist-antagonist combinations.
| Agonist |
Antagonist |
Movement |
|
Biceps brachii—located on the anterior surface of the upper arm |
Triceps brachii—located on the posterior surface of the upper arm |
The biceps brachii flexes the forearm, while the triceps brachii extends
it. |
|
Hamstrings—group of three muscles located on the posterior of the thigh |
Quadriceps femoris—group of four muscles located on the anterior of the thigh |
The hamstrings flex the lower leg. The quadriceps femoris extend the lower
leg. |
|
Flexor digitorum superficialis and flexor digitorum profundus—located in and around the
hand |
Extensor digitorum—located in and around the hand |
The flexor digitorum superficialis and flexor digitorum profundus flex the
fingers and the hand at the wrist while the extensor digitorum extends the
fingers and the hand at the wrist. |
Gross Anatomy of Muscles
Nomenclature for skeletal muscles is based on numerous criteria including: the
location of the muscle, the shape, the orientation, the number of the muscle in
a group, the action it generates and the location of its origin(s) and
insertion(s). The origin is the unmoving, stable connection of the muscle to a
structure, usually a bone. The insertion is the moveable end of the muscle, and
it inserts onto various structures in the body.
Location: Some muscle names indicate the bones or body
region with which the muscle is associated. For example, the frontalis muscle is
located on top of the frontal bone of the skull. The rectus femoris (femur) and
brachioradialis (arm and radius) are also good examples.
Shapes: Muscles with distinctive shapes are often named for the shape. The
trapezius in the neck and back is similar to a trapezoid. You have already
learned about muscles that have “orbicularis” in the name. The size of the
muscle is the source of the names of the muscles of the buttocks: gluteus
maximus (largest), gluteus medius (medium) and gluteus minimus (smallest). There
are also muscles with names that contain brevis (short), longus (long),
lateralis (lateral side or away from midline) and medialis (toward the midline).
Some practice with the names of common muscles will help you with the
descriptions that indicate muscle size or location.
Orientation: The direction of the muscle fibers and fascicles relative to the
midline are also used to describe muscles, such as the rectus (straight)
abdominis or the internal and external oblique (at an angle) muscles of the
abdomen.
Numbers in a group: Some muscles are named according to the number of muscles in
a “group.” One example of this is the quadriceps, a group of four muscles
located on the anterior (front) of the thigh. Other muscle names can give you a
clue as to how many origins a particular muscle has, such as the biceps brachii
or triceps brachii. Bi- indicates that the muscle has two origins and tri-
indicates three.
Action: When muscles are named for the movement they produce, you will find
action words in their name. Some examples are flexor (decreases the angle at the
joint), extensor (increases the angle at the joint), abductor (moves the bone
away from the midline) or adductor (moves the bone toward the midline).
Attachments: The location of a muscle’s orientation and insertion can
also be used in its name. For this classification, the origin is always named first. For
instance, the sternocleidomastoid muscle of the neck has a dual origin on the
sternum (sterno) and clavicle (cleido) and it inserts on the mastoid process of
the temporal bone.
The following table lists important muscle terminology.
| Name |
Definition |
Example |
| Direction
Relative to the Midline of the Body |
| rectus |
straight |
rectus abdominis |
| transverse |
at right angle |
transverse abdominis |
| oblique |
diagonal |
external oblique |
| Relative
Size |
| maximus |
largest |
gluteus maximus |
| medius |
medium |
gluteus medius |
| minimus |
smallest |
gluteus minimus |
| longus |
long |
longus capitis |
| brevis |
short |
extensor carpi radialis brevis |
| latissimus |
widest |
latissimus dorsi |
| longissimus |
longest |
longissimus thoracis |
| magnus |
large |
adductor magnus |
| major |
larger |
rhomboid major |
| minor |
smaller |
rhomboid minor |
| vastus |
huge |
vastus medialis |
| Relative
Shape |
| deltoid |
triangular |
deltoid |
| trapezius |
trapezoidal |
trapezius |
| serratus |
serrated |
serratus anterior |
| rhomboid |
diamond-shaped |
rhomboid minor |
| orbicularis |
circular |
orbicularis oris |
| pectinate |
comb-like |
pectineus |
| piriformis |
pear-shaped |
piriformis |
| platys |
flat |
platysma |
| quadratus |
four-sided and square |
quadratus lumborum |
| gracilis |
slender |
gracilis |
| Action |
| flexor |
decreases the angle at a joint |
flexor digitorum superficialis |
| extensor |
increases the angle at a joint |
extensor digitorum |
| abductor |
moves the bone away from the midline |
abductor pollicis longus |
| adductor |
moves the bone toward the midline |
adductor magnus |
| levator |
elevates a body part |
levator scapulae |
| depressor |
lowers a body part |
depressor labii inferioris |
| supinator |
turns the palm anteriorly |
supinator |
| pronator |
turns the palm posteriorly |
pronator teres |
| sphincter |
decreases the size of an opening |
external anal sphincter |
| tensor |
tenses a body part |
tensor fasciae latae |
| rotator |
rotates a bone around its longitudinal axis |
rotator |
| Number of
Origins |
| biceps |
two |
biceps brachii |
| triceps |
three |
triceps brachii |
| quadriceps |
four |
quadriceps femoris |
Example
Explicit Muscle Anatomy: Axial Muscles of the Face, Neck, and Head
There are more than 600 individually identified skeletal muscles in the human body. An entire
course could be spent just learning the names, origins, insertions and actions of
skeletal muscles and muscle groups of the body. This course presents an example of
integrated muscle units, so that you can appreciate the complex integration of
muscle anatomy and physiology.
Muscles can be classified either as axial or appendicular muscles, depending on
whether they act on bones of the axial or appendicular skeleton, respectively. If we
just look at the axial muscles, we can further divide them into groups on the basis
of location, function, or both. Some of the axial muscles may seem to blur the
boundaries due to the fact that they cross over to the appendicular skeleton. The
muscles of the head and neck would be considered a subgroup of the axial muscles
based on location.
This group can also be divided into those that insert into skin or those that insert
on bones. The muscles in the face create facial
expressions
by inserting into the skin rather than onto bone. These muscles include the
occipitofrontalis, orbicularis oris, buccinators, orbicularis oculi, and corrugator
supercilii among others.
The force generation required for skeletal muscle function occurs at the molecular level. You
can develop a better understanding of the properties of cells and tissues by studying
the molecular mechanisms common to the cells involved:
-
Molecular level — actin and myosin
-
Microscopic level — sarcomere and myofibrils
-
Cell level — myoblasts and myofibers
-
Tissue level — neuromuscular junctions and fascicles
-
Organ level — major skeletal muscles of the body
Myosin is a protein that converts the chemical energy stored in the bonds of
ATP into the kinetic energy of movement. Myosin is the force-generating protein in all
muscle cells, and a coordinated effort among many myosin molecules pulling on actin,
generates force for movement. Myosin molecules have two main structural parts. The tail
of a myosin molecule consists of two polypeptide subunits wound together, whereas the
head is composed of two globular subunits. The tail of a myosin molecule connects with
other myosin molecules to form the central region of a thick filament, whereas the heads
align on either end of the thick filament where thin filaments overlap with the thick
filament. The point at which the head and tail of the molecule meet is flexible and
allows the head to move back and forth. This allows myosin to “walk” and pull on
actin filaments. Actin filaments are made of individual globular
(spherical) protein subunits that assemble linearly into helical (twisted) filaments.
ATP binding to
myosin causes it to release actin, allowing actin and myosin to detach from each other.
After this occurs, ATP is converted to ADP and Pi. The binding site on myosin that
hydrolyzes ATP to ADP is called ATPase. The energy released during ATP
hydrolysis changes the angle of the myosin head into a “cocked” position. The myosin
head is then in a position for further movement, possessing potential energy; however,
ADP and Pi are still attached.
Cross-bridge formation occurs at this point, as actin binds while ADP and Pi are still
bound to myosin. Pi is then released, causing myosin to form a stronger attachment to
actin, and the myosin head moves toward the M line, pulling actin along with it. As
actin is pulled, the filaments move approximately 10
nanometers
toward the M line. This movement is called the power stroke, as the thin
filament “slides” over the myosin and ADP is released during this step.
When the myosin head is “cocked,” myosin is in a high-energy configuration. This energy
is expended as the myosin head moves through the power stroke. At the end of the power
stroke, the myosin head is in a low-energy position. Each myosin molecule may form many
cross-bridges during muscle contraction. The collection of power strokes and
cross-bridges allows collections of individual molecules to generate large forces.
In skeletal muscle, tropomyosin and troponin regulate
contraction by controlling the interaction between actin and myosin filaments.
Tropomyosin acts to block myosin binding sites on the actin filament, preventing
cross-bridge formation and thus preventing contraction in a relaxed muscle. Calcium
triggers muscle contraction by binding to troponin and altering its shape so that
tropomyosin does not block the myosin binding sites on actin, thus allowing muscle
contraction to occur. Calcium is generally an important molecule in muscle function, as
we will discuss later.
Titin, as the name implies, is a very large structural protein in muscle
cells. Unlike actin and myosin, which bundle together to form a multiprotein complex,
titin is a single protein that holds large structures together. Thus, titin is a large,
multifunctional protein (hundreds of times bigger than these other proteins) that forms
an elastic filament. Titin helps align the myosin proteins and allows the muscle cell to
maintain structural integrity by resisting extreme stretching, preventing damage due to
overstretching.
Dystrophin is a protein that helps bind actin to the muscle cell membrane.
Insufficient dystrophin production results in an inability to transfer the force of the
organized actin-myosin contraction to the muscle cell membrane and ultimately to the
tendons. Loss or insufficient production of this molecule causes Duchenne muscular
dystrophy (DMD).
Example
Rigor and Rigor Mortis
At the end of the power stroke, the actin-myosin cross-bridge is still in place
(until ATP binds to the myosin head to change its shape). When energy is depleted,
ATP is no longer available to bind to myosin; without ATP, actin remains bound to
myosin, making both relaxation and further contraction impossible. This state, with
an intact cross-bridge and depleted ATP, is called rigor.
This situation is exemplified during rigor mortis, which occurs after death. Dead
cells can no longer produce ATP. Without ATP, the myosin heads can not detach from
actin. Recall that ATP is required for the myosin to come off of the actin.
Rigor mortis eventually subsides as proteins in the body, including actin and
myosin, degrade.
The fundamental functional unit of muscle is called a sarcomere. One muscle
may contain as many as 100,000 of the repeating sarcomere units. In the sarcomere, the
myofilaments (thick filaments and thin filaments) are organized into parallel units.
Sarcomeres were first identified by imaging (histology), and the nomenclature described
below reflects their microscopic "appearance."

Molecular Organization of Muscle
Myofilaments are organized structures in muscle cells that contain the actin and
myosin. The organized globular proteins of actin in muscle cells form a
thin filament, and bundles of over 200 myosin proteins form a
thick filament. The thick filament myosin heads “walk” along
the actin thin filaments. A single thin filament is composed of 300-400 globular
actins with 40-60 troponin and tropomyosin molecules. A single thick filament is
composed of more
than
200 myosin molecules.
Actin filaments are thin, causing the actin "rope" to appear skinny. The myosin filament
contains many myosins bundled up with all of the head groups sticking out, so
that it looks “fluffy.” That fluffiness makes it look thick.
Sarcomere Anatomy—H Zone, M Line, Z Disc, I Band and A Band

Histological sections of muscle show the anatomical features of the sarcomeres.
Thick filaments, composed of myosin, are visible as the A band of a sarcomere.
Thin filaments, composed of actin, attach to a protein in the Z disc (or Z line)
called alpha-actinin, and they are present across the entire length of the I
band and a portion of the A band. The region where thick and thin filaments
overlap has a dense appearance, as there is little space between the filaments.
This zone where thin and thick filaments overlap is very important to muscle
contraction, as it is the site where filament movement starts. Thin filaments do
not extend completely into the A bands, leaving a central region of the A band
that only contains thick filaments. This central region of the A band looks
slightly lighter than the rest of the A band, and is called the H zone. The
middle of the H zone has a vertical line called the M line, where accessory
proteins hold together thick filaments.
| Microscopically Visible Feature |
Composition |
| A band |
Region of thick-filament myosin proteins |
| H zone |
Central region of the A band with no overlapping actin proteins when
muscle is relaxed |
| I band |
Region of thin-filament actin proteins, with no myosin |
| M line |
M line accessory proteins in center of myosin thick filament
perpendicular to the sarcomere |
| Z disc |
Zig-zag line of Z line proteins and actin binding proteins perpendicular
to the sarcomere |
Cellular Organelles and Structures
Muscle cells contain organelles found in all cells, including nuclei, the
endoplasmic reticulum, mitochondria and the Golgi apparatus. The amount and
organization of organelles and structures is slightly different in muscle cells.
Actin is found inside every cell in the body, but actin is specially organized
within the sarcomeres of muscle cells. Muscle cells also have extremely high
numbers of mitochondria to produce ATP for force generation. Recall that an ATP
molecule is required for one myosin to perform one power stroke.
Click here to review cells and organelles
in the Levels of Organization unit.
Organelles and Structures Specific to Muscle Cells
 This image shows the internal organization of a muscle cell.
This image shows the internal organization of a muscle cell.
 The image is a light microscopic image of skeletal muscle in cross
section showing a group muscle cells, each surrounded by epimysium and
the group surrounded by perimysium. Blood vessels are seen passing
through the image and in cross section on the right. The magnified
image shows cross section of a single cell (fiber) at higher
magnification with many myofibrils visible (small dark circles).
The image is a light microscopic image of skeletal muscle in cross
section showing a group muscle cells, each surrounded by epimysium and
the group surrounded by perimysium. Blood vessels are seen passing
through the image and in cross section on the right. The magnified
image shows cross section of a single cell (fiber) at higher
magnification with many myofibrils visible (small dark circles).
Each skeletal muscle fiber is a single cell produced from the fusion of many
precursor cells. These fused cells are therefore functionally quite large, with
diameters of up to 100 micrometers and lengths of up to 30 centimeters. Compare
this with a cell of the skin which is a cube of 20 micrometers in nearly every
diameter. In muscle fibers, sarcomeres arrange into parallel structures called
myofibrils, so both the Z disc and the M line hold myofilaments
in place to maintain the structural arrangement and layering. Myofibrils are
connected to each other by intermediate, or desmin, filaments that attach to the
Z disc.
There is some special nomenclature associated with these fused cells. Each
skeletal muscle fiber cell has more than one nucleus, which is called
multinucleate. The plasma membrane of this fused skeletal
muscle cell is called the sarcolemma. The muscle interacts with the
nerves that stimulate the muscle at the sarcolemma, and we will describe this
interaction later. Within the sarcolemma is the sarcoplasm, which
is the cytoplasm of the fused muscle fiber. The sarcoplasm has most of the same
components as standard cytoplasm in addition to high levels of the protein
myoglobin, which stores oxygen.
Also, the sarcolemma contains many structures similar to the plasma membrane of
other cells, but it also possesses structures unique to muscle cells. The
sarcolemma has transverse tubules, or T tubules, which are
indentations of the sarcolemma into the interior of the cell along the length of
the muscle cell. The T tubules are filled with extracellular fluid, and they
conduct the action potential from the nerve deep into the interior of the muscle
cells, which can be very large. With T-tubules, nerves can stimulate entire
muscles and muscle groups quickly and effectively. Without T tubules, action
potential conduction into the interior of the cell would happen much more
slowly, causing delays between neural stimulation and muscle contraction,
resulting in slower, weaker contractions.
Inside skeletal muscle fibers is a network of membranous tubules called the
sarcoplasmic reticulum (SR), which is similar to the smooth endoplasmic
reticulum found in other cells. SR tubules are filled with high-calcium fluid,
and they surround each myofibril. Terminal cisternae are dilated
regions of the SR that form on either side of each T tubule extending into the
cell. A grouping consisting of a T tubule, from the outside of the muscle fiber,
and two terminal cisternae, from the inside of the muscle fiber, is called a
triad.
T tubules conduct an action potential along the surface of the muscle fiber into
triads that trigger the release of Ca2+ ions from the nearby terminal cisternae.
This, in turn, triggers muscle contraction when the calcium ions in the
sarcoplasm can bind to the troponin of the sarcomeres.
Skeletal Myocytes—Myoblast Fusion, Myotube Organization in Skeletal Muscle Tissue
and Satellite Cells
Each skeletal muscle fiber is a single skeletal muscle cell, also known as a
skeletal myocyte ("myo-" refers to "muscle" and "-cyte" refers to "cell"), that
is formed from the fusion of precursor cells. As described before, cell fusion
leads to multinucleation of each mature muscle fiber. Each myoblast, the
embryonic cell type that differentiates into muscle, contributes one nucleus
when the muscle fiber is formed during development.
During development, individual myoblasts ("-blast" refers to
"building" … like osteoblasts), migrate to different regions in the body and
then fuse to form a myotube. A myotube is a type of
syncytium,
which is the term used for a group of fused cells. Skeletal muscle cells are
multinucleate because the syncytium
("syn-"
means "same" and "cyt" refers to "cytoplasm") fusion retains
the nucleus of each contributing myoblast. This syncytium leads to the
collective sarcoplasm and sarcolemma, described above.
Mature muscle does not grow by this process. Mature cells can change in size, but
new cells are not formed when muscles grow. Instead, structural proteins are
added to muscle fibers in a process called hypertrophy. The
reverse, when structural proteins are lost and muscle mass decreases, is called
atrophy. Cellular components of muscles can also undergo
changes in response to changes in muscle use.
Although the number of muscle cells is set during development, satellite
cells help to repair skeletal muscle fibers. Satellite cells are
similar to myoblasts in that they are able to divide, fuse and differentiate.
These satellite cells are located outside the muscle fibers and are stimulated
to grow and fuse with muscle cells by growth factors that are released by muscle
fibers under certain forms of stress. Satellite cells can regenerate muscle
fibers to a very limited extent, but they primarily help to repair damage in
living cells. Satellite cells facilitate the protein synthesis required for
repair and growth. If a cell is damaged to a greater extent than can be repaired
by satellite cells, the muscle fibers are replaced by scar tissue in a process
called fibrosis. Because scar tissue cannot contract, muscle that
has sustained significant damage loses strength and cannot produce the same
amount of force or endurance as it could before being damaged.
Cardiac Myocytes
Cardiac tissue is only found in the walls of the heart chambers, where it
provides the muscle contractions required to pump blood throughout the body. At
the tissue level, cardiac muscle is striated (or striped) since, like skeletal
muscle, it has organized sarcomeres. However, cardiac muscle fibers are shorter
than skeletal muscle fibers and usually contain only one nucleus, which is
located in the central region of the cell.


Cardiac muscle fibers are branched, whereas skeletal muscle fibers are
unbranched. This branching allows individual cells to contact several adjacent
cells at specialized cell junctions called intercalated discs.
Intercalated discs are part of the sarcolemma and contain two structures
important in cardiac muscle contraction: gap junctions and desmosomes. Gap
junctions
are tunnels or protein channels in the cell membrane
connecting adjacent cardiac muscle cells, allowing ions involved in electric
currents to move quickly from one cell to the next. This is called
electric coupling, and in cardiac muscles, it allows the quick
transmission of action potentials and synchronized contraction of the entire
heart. Desmosomes are especially strong cell-to-cell junctions that help
maintain structural integrity at the connections between these contractile
cardiac muscle cells.
Cardiac Cell Specialization
There are two specialized types of cardiac cells: the contractile cells and the
pacemaker cells. The contractile cells that produce the force for the beating of
the heart have the capability to beat on their own, but for useful organ level
contractions, the cells must beat as a unit. The stimulus for contraction is
normally provided by the pacemaker cells and this stimulus is passed through gap
junctions to synchronize the signals. These electrically conductive pacemaker
cells are important for electrical stimulation since cardiac muscle cells are
not under voluntary control. Pacemaker cells are spontaneously depolarizing at
set intervals (faster than contractile cells would do on their own), starting a
wave of depolarization that then spreads throughout the heart and triggers
contraction. Because the pacemaker cells are located in the heart, the heart is
said to control its own contraction, which is called
autorhythmicity (or automaticity). Pacemaker cells
depolarize at set intervals, and the heart beats a steady, predictable 60 to 80
beats per minute at rest. These repetitive contractions ensure a constant blood
supply to all body cells.
Smooth muscle tissue is found in many different body systems, including as part of organs in
the digestive, respiratory, and reproductive tracts and in the walls of blood vessels.
Smooth muscle cells are approximately the same size as cardiac muscle cells and also
have only one nucleus. However, smooth muscle cells are not branched and, unlike both
cardiac and skeletal muscle, smooth muscle cells don't have sarcomeres. Smooth muscle
cells form layers that are usually arranged so that one runs parallel to an organ and
the other wraps around it. These two muscle layers then contract in turn, causing
alternating dilation and contraction or lengthening and shortening of the organ, moving
substances through internal passages. This is called
peristalsis
and is displayed in the process of digestion as food moves through the gastrointestinal
tract.
Similar to skeletal and cardiac muscle cells, smooth muscle can undergo hypertrophy to
increase in size. Unlike the other two muscle types, mature smooth muscle cells can also
divide to produce more cells, a process called hyperplasia. This can be
observed in the uterus, which responds to increased estrogen levels by producing more
uterine muscle cells.
Smooth muscle cells also do not possess T tubules and do not have a very extensive
sarcoplasmic reticulum. Smooth muscle has actin and myosin but they are not organized
into sarcomeres, so there are no obvious bands or striations. Instead, actin and myosin
is organized into dense bodies attached to the sarcolemma, shortening the
muscle cell as thin filaments slide past thick filaments. Thin and thick filaments are
aligned in a diagonal pattern across the cell so that contraction produces a twisting or
corkscrew motion, rotating one way as it contracts and the other way as it relaxes.
Cross-bridge formation and filament sliding processes are the same in smooth muscle as
they are in skeletal and cardiac muscle. Actin, myosin, and tropomyosin are all present,
but smooth muscle cells do not possess troponin as their regulatory protein. Instead, a
molecule called calmodulin binds to calcium and activates myosin cross-bridge formation.
There is also a greater ratio of actin to myosin in smooth muscle, meaning that there
are more thin filaments for every thick filament.
Most smooth muscles must function for long periods without rest, so their power output is
relatively low, but contractions can continue without utilizing large amounts of energy.
This occurs because the ATPase in myosin works at a relatively slow rate, meaning that
high levels of ATP are not available for powerful contractions but a steady supply is
produced for sustained contractions. Smooth muscle can also maintain contractions
through a latch state, during which actin and myosin remain locked
together, or latched, in the absence of Ca2+ ions. This does not require ATP, thereby
producing sustained contractions without using energy. This allows smooth muscles to
keep your blood vessels partially contracted for your entire life without them
fatiguing.
Similar to cardiac muscle, smooth muscle is not under voluntary control. In addition to
spontaneous stimulation, smooth muscle can be stimulated by pacesetter
cells that are similar to pacemaker cells and trigger waves of action
potentials in smooth muscle. Smooth muscle can also be stimulated by the autonomic
nervous system or hormones. Neuromuscular junctions are not present in smooth muscle,
but varicosities, enlargements along autonomic nerves, release
neurotransmitters into synaptic clefts. Smooth muscle can respond to a variety of
neurotransmitters to produce different effects at different locations.
Smooth muscle can be divided into two types based on how depolarization and muscle contraction
occur. Single-unit smooth muscle cells contain gap junctions, which allow
the cells to be electrically coupled. Electric couplings allow action potentials to
spread quickly from one cell to the next, permitting coordinated depolarization and
contraction. In this manner, groups of muscle cells act as a single unit, contracting in
unison. This type of smooth muscle is found in hollow organs, including the
gastrointestinal tract, and in the walls of small blood vessels, and it is often
stimulated spontaneously or by stretching, to produce an action potential.
Multiunit smooth muscle cells rarely possess gap junctions, so they are not electrically
coupled. Stimuli for multiunit smooth muscles come from autonomic nerves or hormones but
not from stretching. This type of tissue is found in the walls of large blood vessels,
in the respiratory airways, and connected to hair follicles (to make your hair "stand
up”), among other places.
Skeletal muscle cell contraction occurs after a release of calcium ions from internal
stores, which is initiated by a neural signal. Each skeletal muscle fiber is controlled
by a motor neuron, which conducts signals from the brain or spinal cord to the
muscle.
The following list presents an overview of the sequence of events involved in the contraction
cycle of skeletal muscle:
- The action potential travels down the neuron to the presynaptic axon terminal.
- Voltage-dependent calcium channels open and Ca2+ ions flow from the extracellular
fluid into the presynaptic neuron's cytosol.
- The influx of Ca2+ causes neurotransmitter (acetylcholine)-containing
vesicles to dock and fuse to the presynaptic neuron's cell membrane.
- Vesicle membrane fusion with the nerve cell membrane results in the emptying of the
neurotransmitter into the synaptic cleft; this process is called exocytosis.
- Acetylcholine diffuses into the synaptic cleft and binds to the nicotinic
acetylcholine receptors in the motor end-plate.
- The nicotinic acetylcholine receptors are ligand-gated cation channels, and open
when bound to acetylcholine.
- The receptors open, allowing sodium ions to flow into the muscle's cytosol.
- The electrochemical gradient across the muscle plasma membrane causes a local
depolarization of the motor end-plate.
- The receptors open, allowing sodium ions to flow into and potassium ions to flow out of the
muscle's cytosol.
- The electrochemical gradient across the muscle plasma membrane (more sodium moves in than
potassium out) causes a local depolarization of the motor end-plate.
- This depolarization initiates an action potential on the muscle fiber cell membrane
(sarcolemma) that travels across the surface of the muscle fiber.
- The action potentials travel from the surface of the muscle cell along the membrane of T
tubules that penetrate into the cytosol of the cell.
- Action potentials along the T tubules cause voltage-dependent calcium release channels in the
sarcoplasmic reticulum to open, and release Ca2+ ions from their storage
place in the cisternae.
- Ca2+ ions diffuse through the cytoplasm where they bind to troponin,
ultimately allowing myosin to interact with actin in the sarcomere; this sequence of
events is called excitation-contraction coupling.
- As long as ATP and some other nutrients are available, the mechanical events of contraction
occur.
- Meanwhile, back at the neuromuscular junction, acetylcholine has moved off of the
acetylcholine receptor and is degraded by the enzyme acetylcholinesterase (into
choline and acetate groups), causing termination of the signal.
- The choline is recycled back into the presynaptic terminal, where it is used to
synthesize new acetylcholine molecules.
Anatomy
We stimulate skeletal muscle contraction voluntarily. Electrical signals from the
brain through the spinal cord travel through the axon of the motor neuron. The
axon then branches through the muscle and connects to the individual muscle
fibers at the neuromuscular junction. The folded sarcolemma of the
muscle fiber that interacts with the neuron is called the motor
end-plate; the folded sarcolemma increases surface area contact with
receptors. The ends of the branches of the axon are called the synaptic
terminals, and do not actually contact the motor end-plate. A synaptic
cleft separates the synaptic terminal from the motor end-plate, but
only by a few nanometers.
Communication occurs between a neuron and a muscle fiber through
neurotransmitters. Neural excitation causes the release of neurotransmitters
from the synaptic terminal into the synaptic cleft, where they can then bind to
the appropriate receptors on the motor end-plate. The motor end-plate has folds
in the sarcolemma, called junctional folds, that create a large surface area for
the neurotransmitter to bind to receptors. Generally, there are many folds and
invaginations that increase surface area including junctional folds at the motor
endplate and the T-tubules throughout the cells.
Physiology
The neurotransmitter acetylcholine is released when an action
potential travels down the axon of the motor neuron, resulting in altered
permeability of the synaptic terminal and an influx of calcium into the neuron.
The calcium influx triggers synaptic
vesicles,
which package
neurotransmitters,
to bind to the presynaptic membrane and to release acetylcholine into the
synaptic cleft by exocytosis.
Review the section of this course
about membranes if you need a refresher.
The balance of ions inside and outside a resting membrane creates an electric
potential difference across the membrane. This means that the inside of the
sarcolemma has an overall negative charge relative to the outside of the
membrane, which has an overall positive charge, causing the membrane to be
polarized. Once released from the synaptic terminal, acetylcholine diffuses
across the synaptic cleft to the motor end-plate, where it binds to
acetylcholine receptors, primarily the nicotinic acetylcholine receptors. This
binding causes activation of ion channels in the motor end-plate, which
increases permeability of ions via activation of ion channels: sodium ions flow
into the muscle and potassium ions flow out. Both sodium and potassium ions
contribute to the voltage difference while ion channels control their movement
into and out of the cell. As a neurotransmitter binds, these ion channels open,
and Na+ ions enter the membrane. This reduces the voltage difference between the
inside and outside of the cell, which is called depolarization. As
acetylcholine binds at the motor-end plate, this depolarization is called an
end-plate potential. It then spreads along the sarcolemma, creating an action
potential as voltage-dependent (voltage-gated) sodium channels adjacent to the
initial depolarization site open. The action potential moves across the entire
cell membrane, creating a wave of depolarization.
After depolarization, the membrane needs to be returned to its resting state.
This is called repolarization, during which sodium channels close and potassium
channels open. Because positive potassium ions
(K+)
move from the intracellular space to the extracellular space, this allows the
inside of the cell to again become negatively charged relative to the outside.
During repolarization, and for some time after, the cell enters a refractory period, during which the membrane cannot become
depolarized again. This is because in order to have another action potential,
sodium channels need to return to their resting state, which requires an
intermediate step with a delay.
Propagation of an action potential and depolarization of the sarcolemma comprise
the excitation portion of excitation-contraction coupling,
the connection of electrical activity and mechanical contraction. The structures
responsible for coupling this excitation to contraction are the T tubules and
sarcoplasmic
reticulum (SR). The T tubules are extensions of the sarcolemma and thus carry
the action potential along their surface, conducting the wave of depolarization
into the interior of the cell. T tubules form triads with the ends of two SR
called terminal cisternae. SRs, and especially terminal cisternae, contain high
concentrations of Ca2+ ions inside. As an action potential travels
along the T tubule, the nearby terminal cisternae open their voltage-dependent
calcium release channels, allowing Ca2+ to diffuse into the
sarcoplasm. The influx of Ca2+ increases the amount of calcium
available to bind to troponin. Troponin bound to Ca2+ undergoes a
conformational change
that
results in
tropomyosin
moving on the actin filament. When
tropomyosin
moves, the myosin binding site on the actin is uncovered. This continues as long
as excess Ca2+ is available in the sarcoplasm. When there is no more
free Ca2+ available to bind to troponin, the contraction will stop.
To restore
Ca2+
levels back to a resting state, the excess Ca2+ is
actively transported back into the SR. In a resting state, Ca2+ is
retained inside the SR, keeping sarcoplasmic Ca2+ levels low. Low
sarcoplasmic calcium levels prevent unwanted muscle contraction.
Acetylcholine, often abbreviated as ACh, is a neurotransmitter
released by motor neurons that binds to receptors in the motor end-plate. It is an
extremely important small molecule in human physiology. On the neuron side of the
synaptic cleft, there are typically 300,000 vesicles waiting to be exocytosed at any
time and each vesicle contains up to 10,000 molecules of acetylcholine.
ACh is produced by the reaction of Acetyl coenzyme A (CoA) with a choline molecule in the
neuron cell body. After it is packaged, transported, and released, it binds to the
acetylcholine
receptor on the motor end-plate; it is degraded in the synaptic cleft by the enzyme
acetylcholinesterase (AChE) into acetate (and acetic acid) and choline. The choline is
recycled back into the neuron. AChE resides in the synaptic cleft, breaking down ACh so
that it does not remain bound to ACh receptors, which would interrupt normal control of
muscle contraction. In some cases, insufficient amounts of ACh prevent normal muscle
contraction and cause muscle weakness.
Example
Botulinum's Effect on ACh
Botulinum toxin prevents ACh from being released into the synaptic cleft. With no ACh
binding to its receptors at the motor end-plate, no action potential is produced,
and muscle contraction cannot occur. Botulinum toxin is produced by Clostridium botulinum, a bacterium sometimes found in
improperly canned foods. Ingestion of very small amounts can cause botulism, which
can cause death due to the paralysis of skeletal muscles, including those required
for
breathing.
ATP supplies the energy for muscle contraction to take place. In addition to its direct role in
the cross-bridge cycle, ATP also provides the energy for the active-transport
Na+/K+ and Ca2+ pumps. Muscle contraction does not
occur without sufficient amounts of ATP. The amount of ATP stored in muscle is very low,
only sufficient to power a few seconds worth of contractions. As it is broken down, ATP
must therefore be regenerated and replaced quickly to allow for sustained
contraction.
One ATP moves one myosin head one step. This can generate three picoNewtons (pN) of isometric
force, or move 11 nanometers. Three pN is a very small force—a human bite, generated by
muscle, can generate 500 trillion pN of force. And 11 nm is a very small distance— one
inch has 25 million nanometers.
There are three mechanisms by which ATP can be regenerated: creatine phosphate
metabolism, anaerobic glycolysis, and aerobic respiration.
Creatine phosphate is a phosphagen, which is a compound that can store
energy in its phosphate bonds. In a resting muscle, excess ATP (adenosine triphosphate)
transfers its energy to creatine, producing ADP (adenosine diphosphate) and creatine
phosphate. When the muscle starts to contract and needs energy, creatine phosphate and
ADP are converted into ATP and creatine by the enzyme creatine kinase. This reaction
occurs very quickly; thus, phosphagen-derived ATP powers the first few seconds of muscle
contraction. However, creatine phosphate can only provide approximately 15 seconds worth
of energy, at which point another energy source has to be available.
After the available ATP from creatine phosphate is depleted, muscles generate ATP using
glycolysis. Glycolysis is an anaerobic process that breaks down glucose
(sugar) to produce ATP; however, glycolysis cannot generate ATP as quickly as creatine
phosphate. The sugar used in glycolysis can be provided by blood glucose or by
metabolizing glycogen that is stored in the muscle. Each glucose molecule produces two
ATP and two molecules of pyruvate, which can be used in aerobic respiration
or converted to lactic acid.
If oxygen is available, pyruvic acid is used in aerobic respiration. However, if oxygen is not
available, pyruvic acid is converted into lactic acid, which may contribute
to muscle fatigue and pain. This occurs during strenuous exercise when high amounts of
energy are needed but oxygen cannot be delivered to muscle at a rate fast enough to meet
the whole need. Anaerobic glycolysis cannot be sustained for very long (approximately
one minute of muscle activity), but it is useful in facilitating short bursts of
high-intensity output. Glycolysis does not utilize glucose very efficiently, producing
only two ATP molecules per molecule of glucose, and the by-product lactic acid
contributes to muscle fatigue as it accumulates. Lactic acid is transported out of the
muscle into the bloodstream, but if this does not happen quickly enough, lactic acid can
cause cellular pH levels to drop, affecting enzyme activity and interfering with muscle
contraction.
Aerobic respiration is the breakdown of glucose in the presence of oxygen to produce
carbon dioxide, water, and ATP. Aerobic respiration in the mitochondria of muscles
uses glycogen from muscle stores, blood glucose, pyruvic acid, and fatty acids.
Approximately 95 percent of the ATP required for resting or moderately active muscles is
provided by aerobic respiration. Aerobic respiration is much more efficient than
anaerobic glycolysis, producing approximately 38 ATP molecules per molecule of glucose.
However, aerobic respiration does not synthesize ATP as quickly as anaerobic glycolysis,
meaning that the power output of muscles declines, but lower-power contractions can be
sustained for longer periods.
|
Phosphorylation
of creatine phosphate |
Anaerobic glycolysis |
Aerobic
metabolism |
| Energy source |
Creatine phosphate |
Glucose |
Glucose, pyruvic acid, fatty acids |
| Rate of ATP synthesis |
Very fast |
Fast |
Slow |
| Number of ATPs produced per unit |
1 |
2 |
38 |
| Oxygen required |
No |
No |
Yes |
This combination of different energy sources is important for different types of muscle
activity. As an analogy, a cup of coffee with lots of sugar provides a quick burst of
energy but not for very long. A balanced meal with complex carbohydrates, protein and
fats takes longer to impact us, but provides sustained energy.
Muscles require a large amount of energy, and thus require a constant supply of oxygen and
nutrients. Blood vessels enter muscle at its surface, after which they are distributed
through the entire muscle. Blood vessels and capillaries are found in the connective
tissue that surrounds muscle fascicles and fibers, allowing oxygen and nutrients to be
supplied to muscle cells and metabolic waste to be removed. Myoglobin, which binds
oxygen similarly to hemoglobin and gives muscle its red color, is found in the
sarcoplasm.
Example
Exercise and Energy
After the first few seconds of exercise, available ATP is used up. After the next few minutes,
cellular glucose and glycogen are depleted. After the next 30 minutes, the body's
supply of glucose and glycogen are depleted. After that time, fatty acids and other
energy sources are used to make ATP. That’s why we should exercise for more than 30
minutes to lose weight (i.e. lose fat). Sometimes, time is important.
You have already learned about the anatomy of the sarcomere,with its coordinated actin thin filaments and myosin
thick filaments. For a muscle cell to contract, the sarcomere must shorten in response
to a nerve impulse. The thick and thin filaments do not shorten, but they slide by one
another, causing the sarcomere to shorten while the filaments remain the same length.
This process is known as the sliding filament model of muscle
contraction. The mechanism of contraction is accomplished by the binding of
myosin to actin, resulting in the formation of cross-bridges that generate filament
movement.
When a sarcomere shortens, some regions shorten while others remain the same length. A
sarcomere is defined as the distance between two consecutive Z discs or Z lines. When a
muscle contracts, the distance between the Z discs is reduced. The H zone, the central
region of the A zone, contains only thick filaments and shortens during contraction. The
I band contains only thin filaments and also shortens. The A band does not shorten; it
remains the same length, but A bands of adjacent sarcomeres move closer together during
contraction. Thin filaments are pulled by the thick filaments towards the center of the
sarcomere until the Z discs approach the thick filaments. The zone of overlap, where
thin filaments and thick filaments occupy the same area, increases as the thin filaments
move inward.

The ideal length of a sarcomere to produce maximal tension occurs when all of the thick
and thin filaments overlap. If a sarcomere is stretched past this ideal length, some of
the myosin heads in the thick filaments are not in contact with the actin in the thin
filaments, and fewer cross-bridges can form. This results in fewer myosin heads pulling
on actin, and less tension is produced. If a sarcomere is shortened, the zone of overlap
is reduced as the thin filaments reach the H zone, which is composed of myosin tails.
Because myosin heads form cross-bridges, actin will not bind to myosin in this zone,
again reducing the tension produced by the muscle. If further shortening of the
sarcomere occurs, thin filaments begin to overlap with each other, further reducing
cross-bridge formation and the amount of tension produced. If the muscle
were stretched to the point where thick and thin filaments do not
overlap at all, no cross-bridges are formed, and no tension is produced. This amount of
stretching does not usually occur, as accessory proteins and connective tissue oppose
extreme stretching.

With large numbers of relatively weak molecular motors, we can more easily adjust the force to
meet our needs. Otherwise, we would regularly be producing too little or too much force
for most of our tasks. Also, molecules are only capable of generating small forces based
on their molecular structure.
You have already learned about how the information from a neuron ultimately leads to a
muscle cell contraction.
Revisit previous material for a
review of neuromuscular junctions.
One action potential in a motor neuron produces one contraction. This contraction is called a
twitch. We think of "muscle twitches" as spasms that we can’t control,
but in physiology, a twitch is a technical term describing a muscle response to stimulation. A single
twitch does not produce any significant muscle contraction. Multiple action potentials
(repeated stimulation) are needed to produce a muscle contraction that can produce
work.
A twitch can last from a few milliseconds up to 100 milliseconds, depending on the muscle type.
The tension produced by a single twitch can be measured by a myogram, which
produces a graph illustrating the amount of tension produced over time. When combined
with a plot of electrical signaling, the myogram shows three phases that each twitch
undergoes. The first period is the latent period, during which the action
potential is being propagated along the membrane and Ca2+ ions are released
from the sarcoplasmic reticulum (SR). No tension or contraction is produced at this
point, but the conditions for contraction are being established. This is the phase
during which excitation and contraction are being coupled but contraction has yet to
occur. The contraction phase occurs after the latent period when calcium is
being used to trigger cross-bridge formation. This period lasts from the beginning of
contraction to the point of peak tension. The last phase is the relaxation
phase, when tension decreases as contraction stops. Calcium is pumped out of
the sarcoplasm, back into the SR, and cross-bridge cycling stops. The muscle returns to
a resting state. There is a very short refractory period after the relaxation phase
(Review the previous material about the
physiology of a neuromuscular junction)
A single twitch does not produce any significant muscle activity in a living body. Normal
muscle contraction is more sustained, and it can be modified to produce varying amounts
of force. This is called a graded muscle response. The tension produced in
a skeletal muscle is a function of both the frequency of neural stimulation and the
number of motor neurons involved.
The rate at which a motor neuron delivers action potentials affects the contraction
produced in a muscle cell. If a muscle cell is stimulated while a previous twitch is
still occurring, the second twitch will not have the same strength as the first; it will
be stronger. This effect is called summation, or wave summation, because
the effects of successive neural stimuli are summed, or added together. This occurs
because the second stimulus releases more Ca2+ ions, which become available
while the muscle is still contracting from the first stimulus (the first wave of calcium
ions released). This allows for more cross-bridge formation and greater contraction.
Because the second stimulus has to arrive before the first twitch has completed, the
frequency of stimulus determines whether summation occurs or not.
If the frequency of stimulation increases to the point at which each successive stimulus sums
with the force generated from the previous stimulus, muscle tension continues to rise
until the tension generated reaches a peak point. The tension at this point is about
three to four times higher than the tension of a single twitch; this is referred to as
incomplete tetanus. Tetanus is defined as continuous fused contraction.
During incomplete tetanus, the muscle goes through quick cycles of contraction with a
short relaxation phase. If the stimulus frequency is so high that the relaxation phase
disappears completely, contractions become continuous in a process called complete
tetanus. This occurs when Ca2+ concentrations in the sarcoplasm reach a point
at which contractions can continue uninterrupted. This contraction continues until the
muscle fatigues and can no longer produce tension.
This type of tetanus is not the same as the disease of the same name that is
distinguished by severe sustained contraction of skeletal muscles. The disease, which
can be fatal if left untreated, is caused by the bacterium
Clostridium tetani, which is present in most
environments. The toxin from the bacterium affects how motor neurons communicate and
control muscle contractions, resulting in muscle spasms or sustained contractions, also
known as
“lockjaw.”
Slightly different from incomplete tetanus is the phenomenon of treppe. Treppe
(from the German term for step, referring to stepwise increases in contraction) is a
condition in which successive stimuli produce a greater amount of tension, even though
tension goes back to the resting state between stimuli (in tetanus, tension does not
decrease to the resting state between stimuli). Treppe is similar to tetanus in that the
first twitch releases calcium into the sarcoplasm, some of which will not be taken back
up before the next contraction. Each stimulus afterward releases more calcium, but there
is still some calcium present in the sarcoplasm from the previous stimulus. This extra
calcium permits more cross-bridge formation and greater contraction with each additional
stimulus up to the point where added calcium cannot be utilized. At this point,
successive stimuli will produce a uniform amount of tension.
The strength of contractions is controlled not only by the frequency of stimuli but also by the
number of motor units involved in a contraction. A motor unit is defined as
a single motor neuron and the corresponding muscle fibers it controls. Increasing the
frequency of neural stimulation can increase the tension produced by a single motor
unit, but this can only produce a limited amount of tension in a skeletal muscle. To
produce more tension in an entire skeletal muscle, the number of motor units involved in
contraction must be increased. This process is called recruitment.
The size of motor units varies with the sizes of muscle. Small muscles contain smaller
motor units and are most useful for fine motor movements. Larger muscles tend to have
larger motor units because they are generally not involved in fine control. Even within
a muscle, motor units vary in size. Generally, when a muscle contracts, small motor
units will be the first ones recruited in a muscle, with larger motor units added as
more force is needed.
All of the motor units in a muscle can be active simultaneously, producing a very powerful
contraction. This cannot last for very long because of the energy requirements of muscle
contraction. To prevent complete muscle fatigue, typically motor units in a given muscle
are not all simultaneously active, but instead, some motor units rest, while others are
active, allowing for longer muscle contractions by the muscle as a whole.
The action potentials produced by pacemaker cells in cardiac muscle are longer than those
produced by motor neurons that stimulate skeletal muscle contraction. Thus, cardiac
contractions are approximately ten times longer than skeletal muscle contractions.
Because of long refractory periods, new action potential cannot reach a cardiac muscle
cell before it has entered the relaxation phase, meaning that the sustained contractions
of tetanus are impossible. If tetanus were to occur, the heart would not beat regularly,
interrupting the flow of blood through the body.
Muscle contractions are among the largest energy-consuming processes in the body, which
is not surprising considering the work that muscles constantly do. Skeletal muscles move
the body in obvious ways such as walking and in less noticeable ways such as
facilitating
respiration.
The structure of muscle cells at the microscopic level allows them to convert the
chemical energy found in ATP into the mechanical energy of movement. The proteins actin
and myosin play large roles in producing this movement.
Skeletal Muscle Anatomy
Recall all of the structures of the fused skeletal muscle cell.
If you need to, review organelles and structures specific to the skeletal muscle cells.
Structures analogous to other cell
organelles:
-
Sarcolemma—the
membrane of the fused skeletal
fiber.
-
Sarcoplasm—the
cytoplasm of the fused skeletal
fiber.
-
Sarcoplasmic
reticulum—the
endoplasmic reticulum of the fused skeletal
fiber.
Specialized
structures in muscle
cells:
-
Transverse
tubules (T
tubules)—sarcolemma
tubes filled with extracellular fluid that coordinate conduction in large
muscle
cells.
-
Terminal
cisternae—enlarged
sarcoplasmic reticulum structures store calcium and surround T
tubules.
-
Triad—one
T tubule and two terminal
cisternae.
Skeletal Muscle Fiber Types
There are three main types of skeletal muscle fibers (cells): slow oxidative (SO), which primarily uses aerobic respiration; fast oxidative (FO), which is an intermediate between slow
oxidative and fast glycolytic fibers; and fast glycolytic
(FG), which primarily uses anaerobic glycolysis. Fibers are defined as slow or
fast based on how quickly they contract. The speed of contraction is dependent
on how quickly the ATPase of myosin can hydrolyse ATP to produce cross-bridge
action. Fast fibers hydrolyse ATP approximately twice as quickly as slow fibers,
resulting in quicker cross-bridge cycling. The primary metabolic pathway used
determines whether a fiber is oxidative or glycolytic. If a fiber primarily
produces ATP through aerobic pathways, it is oxidative. Glycolytic fibers
primarily create ATP through anaerobic glycolysis.
Since SO fibers function for long periods without fatigue, they are used to
maintain posture, producing isometric contractions useful for stabilizing bones
and joints, and making small movements that happen often but do not require
large amounts of energy. They do not produce high tension, so they are not used
for powerful, fast movements that require high amounts of energy and rapid
cross-bridge cycling.
FO fibers are sometimes called intermediate fibers because they possess
characteristics that are intermediate between fast fibers and slow fibers. They
produce ATP relatively quickly, more quickly than SO fibers, and thus can
produce relatively high amounts of tension. They are oxidative because they
produce ATP aerobically, possess high numbers of mitochondria, and do not
fatigue quickly. FO fibers do not possess significant myoglobin, giving them a
lighter color than the red SO fibers. FO fibers are used primarily for
movements, such as walking, that require more energy than postural control but
less energy than an explosive movement such as sprinting. FO fibers are useful
for this type of movement because they produce more tension than SO fibers and
they are more fatigue-resistant than FG fibers.
FG fibers primarily use anaerobic glycolysis as their ATP source. They have a
large diameter and possess high amounts of glycogen, which is used in glycolysis
to generate ATP quickly; thus, they produce high levels of tension. Because they
do not primarily use aerobic metabolism, they do not possess substantial numbers
of mitochondria nor large amounts of myoglobin and therefore have a white color.
FG fibers are used to produce rapid, forceful contractions to make quick,
powerful movements. However, these fibers fatigue quickly, permitting them to
only be used for short periods.
Most muscles (organs) possess a mixture of each fiber (cell) type. The
predominant fiber type in a muscle is determined by the primary function of the
muscle. Large muscles used for powerful movements contain more fast fibers than
slow fibers. As such, different muscles have different speeds and different
abilities to maintain contraction over time. The proportion of these different
kinds of muscle fibers will vary among different people and can change within a
person with conditioning.
Skeletal muscles contribute to maintaining temperature homeostasis in the body by
generating heat. Muscle contraction requires energy and produces heat as a byproduct of
metabolism. All types of muscle produce heat, but because of the large amount of
skeletal muscle present in the body, skeletal muscle contributes most greatly to heat
production. This is very noticeable during exercise, when sustained muscle movement
causes body temperature to rise. In cases of extreme cold, shivering produces random
skeletal muscle contractions to generate heat as part of the negative feedback mechanism
of maintaining body temperature.
Example
Malignant Hyperthermia
Our body can use skeletal muscle contractions to maintain body temperature when we
are cold, but excessive contractions can lead to the body overheating to the point
that the body’s metabolic reactions are interrupted. This can occur in a condition
called malignant hyperthermia, which develops in genetically susceptible individuals
who are administered a specific combination of anesthetic agents during surgery. In
these individuals, a drastic increase in skeletal muscle calcium leads to sustained
contractions and heat generation. Because
the
individuals
are anesthetized, they have little ability to cool themselves. If proper
interventions are not administered, they will die due to a greatly increased body
temperature. Because this condition is genetic, patients are asked prior to surgery
if there is a family history of such problems occurring.
Physical training alters the appearance of skeletal muscles and can produce changes in muscle
performance. Conversely, a lack of use can result in decreased performance and muscle
appearance. As
you
learned
earlier, mature muscle cells grow from
hypertrophy,
not cell division. The loss of structural proteins and muscle mass occurs during
atrophy. Cellular components of muscles can also undergo changes in response to changes
in muscle use.
Example
Sarcopenia
Although atrophy due to disuse can often be reversed with exercise, muscle atrophy
that
comes with age is irreversible. This is why even highly trained
athletes succumb to declining performance with age, although extensive training may
slow the decline. This is especially noticeable in sports that require an explosion
of strength and power over a very short period of time. Examples of these kinds of
sports include sprinting, competitive weight lifting, gymnastics and diving. The
effects of age are less noticeable in endurance sports such as marathon running or
long-distance cycling. Age-related muscle atrophy is called sarcopenia. As muscles
age, muscle fibers die, and they are replaced by connective tissue and adipose
tissue. Because those tissues cannot contract as muscle can, muscles lose the
ability to produce powerful contractions. The decline in muscle mass causes a loss
of strength, including strength required for posture and mobility. This may be
caused by a reduction in the proportion of FG fibers that hydrolyse ATP quickly to
produce short, powerful contractions. Muscles in older people sometimes possess
greater numbers of SO fibers, which are responsible for longer contractions and do
not produce powerful movements. There may also be a reduction in motor units,
resulting in fewer fibers being stimulated and less muscle tension being
produced.
Example
Anabolic Steroids
Some athletes attempt to boost their performance by using various agents that may enhance
muscle performance. Anabolic steroids are one of the more widely known agents used
to boost muscle mass and increase power output. Anabolic steroids are a form of
testosterone, a male sex hormone that stimulates muscle formation, leading to
increased muscle mass. They have been used by athletes in many sports, but sprinting
is one sport in which the effects of steroids are readily apparent. Because a
100-meter dash
can
last
less than 10 seconds, incredible amounts of power need to be created by the muscles.
Increasing the muscle mass increases the numbers of actin and myosin cross-bridges,
increasing the power that can be produced by a muscle, which provides a competitive
advantage in a sport measured in
hundredths
of seconds. Similarly, creatine has become a substance used by some athletes to
increase power output. Because creatine phosphate provides quick bursts of ATP to
muscles in the initial stages of contraction, increasing the amount available to
cells is thought to produce more ATP and therefore increase explosive power output.
However, both creatine and steroids are banned in sports and they can be extremely
harmful to other systems of the body as well as
to
long-term muscle health.
Slow fibers are predominantly used in endurance exercises that require little force but involve
numerous repetitions. The aerobic metabolism used by slow-twitch fibers allows them to
maintain contractions over long periods. Endurance training modifies these slow fibers
to make them even more efficient by producing more mitochondria to enable more aerobic
metabolism and more ATP production. Endurance exercise can also increase the amount of
myoglobin in a cell, as increased aerobic respiration increases the need for oxygen.
Myoglobin is found in the sarcoplasm and stores oxygen. Endurance training can also
trigger the formation of more extensive capillary networks around the fiber, a process
called angiogenesis, to supply oxygen and remove metabolic waste. To allow
these capillary networks to supply the deep portions of the muscle, muscle mass does not
greatly increase, maintaining a shorter distance for the diffusion of nutrients and
gases. All of these cellular changes result in the ability to sustain low levels of
muscle
contraction
for greater periods of time without fatiguing.
Example
EPO
Endurance athletes also engage in drug use, but instead of trying to add muscle mass or
produce power, they focus on substances that increase muscle endurance and reduce
fatigue. This includes trying to boost the availability of oxygen to muscles to
increase aerobic respiration by using substances such as erythropoietin, or EPO. EPO
is a hormone that triggers the production of red blood cells, which carry oxygen in
the blood. This oxygen can then be used by muscles for aerobic respiration. Human
growth hormone is another commonly used agent, and although it can facilitate
building muscle mass, its main role is to promote the healing of muscle and other
tissues after strenuous exercise. Increased growth hormone allows for faster
recovery after muscle damage, reducing the rest required after exercise, and
allowing for more sustained high-level performance. As with creatine and steroids,
these substances are harmful to the body and can negatively impact the homeostasis
of other systems.
As discussed previously, there are a number of proteins that are important in
regulating and carrying out the contractile process. Thus there are genetic
disorders that lead to altered protein formation and dysfunction of the muscular
system. Such dysfunction tends to affect many body systems, of which the
respiratory system may be most important. Without the ability to appropriately
contract the skeletal muscles of respiration, people cannot live very long. The
skeletal muscular system is also very dependent upon a constant supply of ATP.
Like many other systems, this requires the intracellular pathways to convert
glucose into energy.
Example
Beriberi
Thiamine (vitamin B1) is a necessary cofactor in the production of ATP from glucose.
Deficiencies in thiamine and other vitamins can lead to extreme muscle weakness as
well as neurological problems. Thiamine deficiency leads to a disease called
beriberi, which was common in people who ate white rice as their major food source.
Beriberi can affect skeletal and cardiac muscles as well as the nervous system.
Eating a balanced diet and the increase of vitamin-enriched foods have eliminated
beriberi from developed countries, but many disorders of vitamin deficiency exist in
developing areas.
Think of all of the activities you do that require function of your muscular system. These
include walking, chewing, swallowing, breathing, talking, etc. Dysfunctions of this
system (such as
weakness
or hypertonia) can prevent the body from carrying out any of these activities normally.
This in turn can affect many other systems. For example, if we can’t bring in
appropriate nutrients and oxygen, every other system in the body would be affected, as
they are all dependent on fuel sources.
Muscles require a large amount of energy and thus require a constant supply of oxygen and
nutrients. Blood vessels enter muscle at its surface, after which they are distributed
through the entire muscle. Blood vessels are found in the connective tissue that
surrounds muscles, allowing oxygen and nutrients to be supplied to muscle and metabolic
waste to be removed. The epimysium houses the arteries and veins that allow blood to
enter and exit muscle. The blood vessels then branch into the epimysium to serve each
fascicle and then further branch in the endomysium to form a capillary network that
contacts each muscle fiber. The flexible, extensively branched capillary networks are
able to withstand muscle contractions without being damaged.
Skeletal muscles are under neural control, which means that nerves must be present for
the muscle to function. The epimysium contains nerve fibers that branch through the
perimysium and epimysium to connect neurons to individual muscle fibers.
Although this unit
has
primarily
focused
on skeletal muscle,
don't
forget about cardiac and smooth muscle, both of which play vital roles in the
cardiovascular system (and smooth muscle in other systems as well). Your heart needs to
continuously contract in order to keep blood flowing to your organs, and in turn, supply
them with needed oxygen and nutrients. But smooth muscle also plays a vital role. It is
found in the walls of all blood vessels except for capillaries, and its function is to
dilate or constrict, depending on the needs of the body and the downstream tissues. For
example, if the skeletal muscles in your legs increase their metabolic rate because you
start running, smooth muscle cells in the arteries feeding these muscles will relax.
This dilates these arteries, allowing for a greater blood flow to the muscles of the
legs. In fact, arteries to other regions (such as the
gastrointestinal
tract) may partially constrict in an effort to shunt more blood toward the legs. It is
these smooth muscles that play a vital role in sending higher amounts of blood to where
it is needed most. Skeletal muscles also play a role within the cardiovascular system.
The heart acts as the pump to move blood out to the body cells, but the skeletal muscles
assist with the movement of blood back to the heart. Because of valves in the veins that
prevent back
flow, the contraction of skeletal muscles around the major veins
helps
move the blood from one compartment to the next, slowly returning blood back to the
heart.
Muscles are extremely important in the digestive system as well. The muscles of our mouth,
tongue and jaw are responsible for biting and chewing our food. Within our digestive
tract, sphincters located throughout the digestive system are responsible for
compartmentalizing the digestive processes within discrete organs. We are most familiar
with the anal sphincter that we are able to control to excrete waste from the digestive
system. Also, throughout the digestive system, smooth muscle helps generate small forces
to mix food in the stomach and help maintain movements throughout the digestive
process.
The integumentary system is one of the main protective systems of the human body. It’s
not a commonly known system. It contains your skin, hair, nails, and several glands. Did
you know your skin is the largest organ in your body? It makes up approximately 7% of
your body weight! The skin is also known as your integument or covering. Have you ever
heard the expression that “beauty is only skin deep”? It’s an interesting statement
because everything you see on another person’s appearance is dead! The layer of skin and
hairs that are visible are actually dead epithelial cells!
The skin is actually formed from two layers: the superficial
epidermis layer which is
composed of epithelial tissue and the deeper connective tissue layer
known as the dermis. The hypodermis is below the dermis and composed of
loose connective tissue.
When you give a subcutaneous (or sub-Q) injection you inject the drug
into the
hypodermis layer. Subcutaneous means below the cutaneous membrane
which is another term
for your skin. One medication commonly given via this method is
insulin.
Let’s test your knowledge on some common facts and myths of the integumentary system.
Let’s check out the integumentary system in action in the following case study.
You and your friend, Tori, are baking cookies. It’s the end of the semester and you both
have been craving chocolate chip goodness. While the cookies are in the oven, you and
Tori decide to study your anatomy and physiology. You are currently studying the
integumentary system. The timer goes off and Tori jumps up, very excited, grabs a dish
towel and pulls the cookie sheet out of the over. “OW!” Tori yells – as she drops the
cookie sheet onto the table. “I just burned my hand on the cookie sheet”.
You reply, “Quick, rinse it under cold water, I think that is supposed to stop the
burn.”
“It really hurts!” Tori exclaims while running her hand under the cool water from the
faucet.
“Let me see your hand” you tell Tori.
When she shows you, you notice a couple blisters beginning to form on her fingers and
palm. “I think you burned through your epidermis layer and maybe to the dermis. Don’t
blisters mean you have a second degree burn?” you ask her.
“What does that mean?” Tori asks. “Should I go to that prompt care place?”
“Let’s look it up, I think if you keep it clean you will be ok. There are several layers
of cells in the epidermis, aren’t there?” you ask. “Our textbook says if the skin
blisters it is a sign of a second degree burn. These burns don’t typically damage the
lower layer, the dermis.”
“It’s a good thing it didn’t burn through the dermis, then I would be more prone to other
infections, wouldn’t I? Didn’t we read about the skin protecting us from microbes around
us?” Tori asks?
The integumentary system is vital for our body’s homeostasis. As we saw with Tori, the
skin is an important barrier for protection. If the skin is damaged from a burn, or
torn, it opens up an entry point for microbes to enter our body. This is why we always
wear protective gloves when handling body fluids. The gloves protect us in case there
are any openings in our skin. It’s better to be extra cautious about this. Our epidermis
is multi-layered to make it better suited for protection. If Tori’s burn had penetrated
deeper it also would have increased fluid loss and dehydration. The skin functions as a
cover to keep interstitial fluids inside rather than seeping out. Every day we lose a
small amount of water through our skin and our lungs from breathing. This water loss is
termed insensible perspiration and is accelerated when in dry air. Our cells in the
epidermis produce a protein known as keratin that helps to decrease water loss from our
skin. If skin becomes dry, it cracks and creates an opening in our defenses. When you
think of maintaining homeostasis, protection is probably one of the first processes you
think of and the skin is vital for this.
The integumentary system is also crucial for body temperature regulation. When we are too
warm, blood vessels going to the skin open up (vasodilate) and more blood flows to the
surface of the skin to release heat to cool the body. Our skin turns pink or red when
this happens. If body temperature continues to rise, our sweat glands become active and
we sweat to cool off (this is termed sensible perspiration). When we are cold, the blood
vessels shrink (vasoconstrict) to decrease blood flow, and consequently heat loss, from
our skin. Our skin might turn blue from the decrease in blood flow. We also have a layer
of body hair covering our body which insulates and helps us retain heat. It’s a good
idea to wear a hat when in cold weather to reduce heat loss from our head!
Another function of the skin, isn’t commonly known. The skin produces vitamin D in
response to sunlight or UV radiation. Vitamin D is a very important vitamin, it’s
crucial for calcium absorption from our food, and has recently been shown to have
anti-cancer properties. If our vitamin D levels fall, this could impact our skeletal
system by decreasing the absorption of calcium, and consequently the amount that gets to
our bones.
Our skin is also tied into our nervous system. One of our main senses – touch is
dependent on numerous receptors located in our skin. We can feel and process information
regarding pressure, temperature, light touch, and pain through our skin. If Tori didn’t
feel pain when she touched the hot cookie sheet, she wouldn’t have dropped it to reduce
the damage from the heat.
Clearly, the integumentary system is more involved in our functioning than a clear skin
and gorgeous hair!
The integumentary system is composed not only of the skin, but also nails, glands, and hair.
The most numerous component of the integumentary system is the integument or skin. The
skin contains the superficial epidermis, which consists of epithelial tissue, and the
deeper dermis which is formed from dense irregular connective tissue. The epidermis
contains nerve endings for pain, which is why Tori felt the pain of the burn. The
epidermis is avascular, which means it doesn’t have blood capillaries. Nutrients get to
the epidermis from the vascular dermal layer. Only those cells closest to the dermis are
able to receive the nutrients and these cells have rapid mitotic rates. The cells in the
epidermis migrate to the surface, and then are shed daily. They are constantly being
replaced by the cells deeper. The dermis contains the blood vessels, sweat and oil
glands. The dermis also has receptors for touch. Below the dermis is the hypodermis
layer. This is the fatty layer that anchors the skin to your body. The hypodermis is
technically not part of the integumentary system.
The skin also contains sweat and oil (sebaceous) glands. Sweat glands release sensible
perspiration to cool us when we overheat. Sweat is mostly water but also contains
electrolytes and a waste product known as urea. Urea is one of the main components of
urine too! Sebaceous glands produce oil, otherwise known as sebum. Sebum and sweat form
a chemical barrier on our skin to decrease bacterial growth on our skin.
Hair and nails are additional structures associated with the integumentary system. Body
hair takes up space to compete with pathogens for room on our skin. Body hair also
insulates us. Did you know that there are approximately 100,000 hairs on your head and
30,000 in a man’s beard? Fingernails and toenails provide leverage and protection when
we grab and manipulate objects.
The epidermis contains the pigment melanin, which protects our cells from UV radiation.
Melanin is also responsible for our hair, skin and eye color. Keratin was previously
mentioned and is important for decreasing water loss from our skin. Many skin lotions
contain keratin to prevent dry skin. Another important protein is collagen. Collagen
provides strength to our skin. Collagen has a white appearance and often when the skin
heals extra collagen is placed at the site. Sometimes this results in a white scar.
When you study the integumentary system, you will learn about the chemical and cellular
components which work together to maintain the integrity of the system. As consumers, we
spend a lot of money on products meant to make our skin and hair look better, younger,
and healthier. As you read through this topic, think about whether those products really
can make a difference or if they are just the result of well-played marketing
campaigns!
The skin is made up of two mutually dependent layers that are distinguished based on
their structure and location. These layers – the epidermis and the dermis – contain a
variety of structures, including blood vessels, hair follicles, and sweat glands.
Beneath the dermis lies the hypodermis (subcutis). It is composed mainly of fatty
tissue.
The most superficial layer, the epidermis, is composed of stratified
squamous epithelia that are keratinized at the outermost surface, melanocytes, immune
cells (Langerhans that modulate immune response) and sensory receptors (Merkel cells
that detect light touch). The function of the epidermis layer is "protection." The
keratinocytes and immune cells help protect the skin.
The dermis lies beneath the epidermis and is composed of two layers of
connective tissue: a loose layer (papillary) and a dense irregular layer (reticular).
Both layers of the dermis contain connective tissue components (collagen, elastin,
fibroblasts), plus blood vessels, sensory receptors and lymphatics. The dermis is a
"functional" layer. The dermis is connective tissue that can stretch and retract because
of the strong and elastic extracellular matrix. The dermis also contains nerves.
Beneath these two layers lies the hypodermis, composed of loose connective tissue
(adipose and areolar). The hypodermis is the "connection" layer. It connects the
integument (epidermis and dermis) to organs and muscles in the body. This layer contains
adipose tissue and connective tissue as well as blood vessels, nerves and immune cells.
The levels of organization of the skin and its accessory
structures
are listed below.
You
will
be exploring these further in the coming pages.
-
Molecular level – keratin, melanin and vitamin D
-
Microscopic level – stem cells and skin cells
-
Tissue level – epithelial and connective tissue
-
Organ level – skin, consisting of the epidermis, dermis, and hypodermis, as well as hair, nails and glands
Keratin
Keratin is a group of fibrous proteins that give hair, nails, and skin their tough,
water-resistant properties. Keratins are filaments formed from
the
polymerization of intermediate filament
proteins. In addition to intra- and intermolecular hydrogen
bonds, keratins have large amounts of the sulfur-containing amino acid cysteine,
which forms disulfide bridges that confer additional strength.
Keratins are an excellent example of protein assembly. Keratins have an alpha-helix secondary
structure in the central rod domain. Two keratin proteins then come together and
the helices wind around themselves to form a
quaternary
structure of a coiled-coil dimer. These dimers then assemble into
protofilaments
and then filaments. The twists of twists are similar to fibers inside of ropes.
However, keratins aren't twisted by machines; they self assemble based on their
primary
structure.
Melanin
Melanin is a class of
photopigment
("photo,"
meaning
"light,"
and
"pigment," meaning
"colored
material")
with a molecular structure that allows it to absorb UV
(ultraviolet)
radiation from the sun. Melanin transforms the energy from the radiation into
harmless heat, and melanin prevents the indirect DNA damage from the sun that is
responsible for many skin cancers. Melanin also gives skin, hair and
eyes
their color. Melanin is produced by specialty cells called melanocytes, inside
special vesicles called melanosomes. About 10 days after initial sun exposure,
melanin synthesis peaks, which is why pale-skinned individuals tend to suffer
sunburns of the epidermis initially.
Example
Albinism
Albinism
is a genetic disorder that affects (completely or
partially) the coloring of skin, hair, and eyes. The defect is primarily due
to
the inability of melanocytes to produce melanin.
Individuals with albinism tend to appear white or very pale due to the lack
of melanin in their skin and hair. Melanin protects the DNA of skin cells
from the harmful effects of UV rays from the sun. Individuals with albinism
need more protection from the sun and must limit their outdoor activities.
Treatment of this disorder usually involves addressing the symptoms.
The epidermal layer of human skin synthesizes the
precursor
to vitamin
D
when exposed to UV radiation. In the presence of sunlight, an isomer
of vitamin D3, cholecalciferol, is synthesized from a derivative of the
steroid cholesterol in the skin. The liver converts cholecalciferol to calcidiol, which
is then converted to calcitriol (the active chemical form of the vitamin) in the
kidneys. Vitamin D, which is really a hormone, is essential for normal absorption of
calcium and phosphorous,
which
are required for healthy bones. In the present day, this hormone is added as a
supplement to many foods including milk and orange juice, compensating for the need for
sun exposure.
In addition to affecting bone health, Vitamin D is essential for general immunity against
bacterial,
viral
and fungal infections. Recent studies are also finding a link between insufficient
vitamin D and cancer.
Example
Rickets
Rickets is a disease of bone deterioration often caused by insufficient vitamin
D,
leading to insufficient calcium absorption. Children are especially prone to effects
from this vitamin deficiency due to their rapid bone turnover and formation. In
adults, the deficiency of vitamin D is called osteomalacia. Typically rickets
is
found in developing countries where malnutrition would prevent proper supply of
calcium and dietary vitamin D.
The epidermis (or epithelial layer) is stratified squamous epithelia, composed of
four to
five
layers (depending on body region) of epithelial cells. The top layers of the epidermis
are made up of keratinocytes, which are cells containing the protein
keratin. The keratinocytes on the most superficial layer of the
epidermis are dead, and periodically slough away, being replaced by cells from the
deeper layers. As keratinocytes move superficially from the deeper layers, they lose
cytoplasm and become flattened, allowing for many layers in a relatively small
space.
Basal cells are an example of tissue-specific stem cells,
meaning they can turn into a variety of cell types found in that tissue. Under normal
conditions, daughter basal cells most commonly replace lost keratinocytes.
The deepest layer of the epidermis and the most superficial layer of the dermis give out
projections that interlock with each other (like Velcro) and strengthen the bond between
the epidermis and the dermis. The projections originating in epithelial cells of the
bottom layer of the epidermis are called desmosomes,
and the ones originating in the dermis are called dermal
papillae. Think of the projections as a formation of folds of cellular
matter. The greater the fold, the stronger the connections made.
Merkel cells are sensory receptors that detect light touch. They form
synaptic connections with sensory nerves that carry touch information to the brain.
These cells are abundant on the surface of the hands and feet.
Melanocytes are cells in the bottom layer of epidermis that produce the
pigment melanin, which gives hair and skin its color. Individuals whose
melanocytes produce more melanin have darker skin color. Cellular extensions of the
melanocytes reach up in between the keratinocytes.
Dendritic or Langerhans cells are tissue macrophages
that contribute to the immune function of
the skin. They engulf foreign organisms and signal to the immune system. Since the skin
is in constant contact with the environment, it is important to have immune cells to
help destroy any pathogens that might get past the cell barrier of the epidermis.
Example
Eczema
Eczema
is an allergic reaction that manifests as dry, itchy patches of
skin that resemble rashes. Normally it is useful to have immune cells in the skin,
but they can also lead to dysfunction if they become overactive. In eczema, this
excess
activity may be accompanied by swelling of the skin, flaking, and in severe cases,
bleeding. Other allergic (immune-mediated) reactions include hives. Symptoms of
these conditions are usually managed with moisturizers and topically with
corticosteroid or
antihistamine
creams that reduce the inflammatory immune response.
Cancer in general is initiated because of DNA damage that accumulates in a
particular cell over time. Exposure to UV rays from the sun can ultimately lead to
mutations in the genomes of various skin cells. Accumulation of genetic mutations over a
period of
time,
in addition to other possible
causes,
can trigger cells to grow out of control. The normal signals that control cell divisions
(even stem cells have these controls) are
lost,
and cells grow to form a tumor, or mass of cells. While some tumors are
benign (stay in one place), some produce cells that dislodge from the tumor and
establish tumors in other areas of the body. In other words, they
metastasize and form secondary tumors.
Basal Cell Carcinoma
Basal cell carcinoma is caused by mutations that lead to lack of control over
the
growth
of stem cells located in
the
stratum basale. It is the most common form of all cancers that
occur in the United States. The head, neck, arms, and back are most
susceptible,
due to long-term sun exposure. Although UV radiation is the main culprit,
exposure to other agents, such as electromagnetic radiation or carcinogenic
chemicals, can also lead to this type of cancer. Injury to the skin due to open
sores, tattoos, burns, etc. may be predisposing factors as well. Basal cell
carcinoma usually starts as an uneven patch, bump, growth, or scar on the skin
surface and responds best to treatment when caught early. Complete surgical
excision of the lesion usually cures this form of cancer.
 Of skin cancers, about
80
percent
are basal cell carcinoma,
16
percent
are squamous cell carcinoma, and
four
percent
are melanoma.
Of skin cancers, about
80
percent
are basal cell carcinoma,
16
percent
are squamous cell carcinoma, and
four
percent
are melanoma.
Squamous Cell Carcinoma
Squamous cell carcinoma is the second most common skin
cancer,
and affects the keratinocytes. Lesions are usually scaly and red and are most
common on the scalp, ears, and hands. If not removed, they can metastasize.
Surgery and radiation are used to cure squamous cell carcinoma.
Melanoma
Melanoma affects melanocytes, the pigment-producing cells in the
epidermis. It is the most fatal of all skin cancers, as it is highly metastatic
and can be difficult to detect before it has spread. Melanomas usually appear as
asymmetrical brown and black patches with uneven borders and a raised surface.
Treatment typically involves surgical excision and immunotherapy.
The skin contains many tissue types. The epidermis is classified as epithelial
tissue composed of stratified squamous epithelia. The dermis is made of
different types of connective tissues including areolar and dense irregular connective
tissue, and histiocytes (tissue macrophages). The hypodermis contains areolar connective
tissue, adipose tissue, and glands.
The epidermis is mainly made up of stratified (layered) squamous (flat) epithelial cells.
Epithelial cells found in the different layers of the epidermis have different shapes.
Stratification (layering) is important in the epithelial tissue of the integumentary
system, which forms a barrier. Epithelial cells found in other systems have other
surface cell
shapes,
including cube-like (cuboid) and column-like (columnar) in
a
single layer (simple) or multiple cell layers (stratified).
The epidermis contains several cell types of different
origins,
including keratinocytes
(95
percent
of the cells are keratinocytes), melanocytes, Langerhans cells and Merkel cells. The
epidermis does not contain blood vessels or nerves, but Merkel cells provide signals to
the sensory neurons below this layer in the dermis. Since there are no blood vessels,
living cells of the epidermis are nourished by diffusion from the capillaries of the
dermal
papillae
below.
While the epidermis is composed of epithelial cells, the dermis is composed of connective
tissue. The dermis connects the epidermis to the hypodermis and provides
structure and elasticity
from
collagen and elastin fibers. These proteins are made by fibroblasts found in the dermis.
Collagen and elastin work together: collagens provide strength; elastins, as the name
implies, are elastic and allow for distension. The skin must remain strong to protect
you from abrasions and other cuts. However, the skin also needs to
be able to
deform and, hopefully, return to its original shape.
Example
Wrinkles
The permanent folds in the skin caused by a lack of
"retraction"
are called wrinkles. Wrinkling in the skin is caused by habitual facial expressions,
aging, sun damage, smoking, poor hydration, and various other factors. Wrinkling is
a surprisingly complex phenomenon. Primarily, sun or other damage to the skin causes
a breakdown in collagen and elastin as well as a breakdown in the replacement of
these extracellular matrix proteins in the dermis.
Example
Stretch marks
Stretch marks usually accompany rapid weight gain during puberty and pregnancy as well as
during the rapid gain of muscle associated with weight lifting. When the dermis (and
possibly the hypodermis) is stretched beyond its limits of elasticity, the skin
stretches to accommodate the excess pressure. In some cases, the dermis can’t
adequately fill in over the stretched areas and
may
actually
tear,
causing the epidermis to become thin and making the underlying blood vessels more
visible. They initially have a reddish hue, but lighten over time as the tissue
repairs itself and heals. Other than for cosmetic reasons, treatment of stretch
marks is not required. They occur most commonly over the hips and abdomen.
Skin contains different types of melanin, including
pheomelanin,
which is reddish, and
eumelanin,
which is dark brown. The amount and types of melanin produced is under genetic control.
The combinations of the different types of melanin result in the wide range of skin
colors
and tones seen in humans. While the number of melanocytes do not vary
too much between individuals, the skin tone depends on the amount and type of melanin
produced by these cells. If a person has more eumelanin producing
melanocytes,
that person would have darker skin color.
The
predominance
of pheomelanin is responsible for the reddish coloration of hair in some
individuals.
Exposure to the sun causes melanin to build up,
because
sun exposure stimulates keratinocytes to secrete a peptide hormone that in turn
stimulates
melanocytes into producing melanin. The melanocytes produce and then transfer the
melanin to keratinocytes. This
buildup
of melanin results in the darkening of the skin, or a tan. Increased melanin also
protects the skin’s DNA from UV ray
damage to
some extent,
although
even very dark-skinned individuals can get skin cancer.
Melanin
synthesis does not peak until
about
10 days after initial sun
exposure,
which is why pale-skinned individuals tend to suffer sunburns of the epidermis
initially.
Individuals
with darker
skin can also get sunburns, but are more protected than
light-pigmented individuals.
Melanosomes, the cellular organelles that contain
melanin,
are temporary structures and are eventually destroyed by fusion with
lysosomes, which makes tanning impermanent. Too much sun exposure can
eventually lead to
wrinkling of
the skin due to destruction of the cellular structure of the
skin,
and in
severe cases
it
can cause sufficient DNA damage to result in skin cancer.
An
uneven distribution of melanocytes in the skin
results
in the appearance of freckles.
Sunscreen and UVA and UVB Radiation
"UV"
is an abbreviation for "ultraviolet."
Ultraviolet
radiation
is
a portion of the electromagnetic spectrum between visible light and x-rays. The
sun emits two ranges of
ultraviolet
light that penetrate earth's
atmosphere:
UVA and UVB. UVB radiation is partially absorbed by the ozone
layer,
but some still reaches the surface of the Earth. UVA radiation is not
filtered,
and all of it reaches the surface of the Earth. One of the functions of our
integumentary system is to protect us from this
UV
radiation. Exposure to this radiation can cause skin damage and cancer.
Sunscreens are designed to protect our skin from UV radiation. Sunscreens with a
sun protection factor (SPF) of 15 or more provide good protection from UVB
radiation. To be protected from UVA
radiation,
you must have a “broad-spectrum” product.
The skin, the integumentary system's organ, is composed of the epidermis (epithelial tissue)
and dermis (connective tissues), with an
underlying
hypodermis that is technically not part of the skin organ. Several layers of
keratinocytes at the surface form the epidermis. The topmost layer is dead and sheds
continuously. It is progressively replaced by stem cells
that
divide in the basal layer (stratum basale). The dermis connects the epidermis to the
hypodermis and provides strength and elasticity due to the presence of collagen and
elastin fibers. The hypodermis is the name for the layer of connective tissue that
connects the dermis to the underlying organs. It also harbors adipose tissue for fat
storage. Let's look at the structure and function of these parts of the skin organ in
detail.
The epidermis (or epithelial layer) is made up of four or five distinct layers
(strata), depending on the region of the body. From deep to superficial, they are named
the stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum
corneum. The stratum lucidum is unique to areas like the palms of the hand (palmar
surfaces) and soles of the feet (plantar surfaces), where the skin is thicker than
it is in
the rest of the body. The stratum basale is made up of the many cell
types already discussed, including basal cells, melanocytes, Langerhans cells and Merkel
cells. As you look at the more superficial layers, you see that they become mostly (or
completely) composed of keratinocytes, which protect and waterproof the body. As the
cells are pushed
superficially
(toward the surface) they make keratin. As the
cells begin to fill with keratin, they become
increasingly
impervious to water,
and it
becomes
more difficult for osmosis and diffusion to occur inside the cell. In addition, as cells
enter each superficial layer
(further
away from the
dermis,
which contains
the
blood
supply),
the distance
across
which oxygen and other nutrients must diffuse increases, making it harder for the cells
to get the nutrients they need. The keratinocytes in the stratum corneum
(the
most superficial layer) are usually inert, or dead, and periodically
slough away, being replaced by cells from the deeper layers.

Stratum Basale
The stratum basale (also called stratum germinativum) is the
deepest epidermal layer and attaches the epidermis to the basal lamina, below
which lie the layers of the dermis. The stratum basale is primarily made up of a
single layer of basal cells. These cells are considered to be stem cells. The
function of this layer is to divide to replicate the cells that are lost from
the surface. The daughter cells then differentiate into keratinocytes. Merkel
cells and melanocytes
are
also
dispersed among the basal cells in
the
stratum basale.
Fingerprints form in the growing fetus where the basal cells of the stratum basale meet the
connective tissue of the underlying dermal layer (papillary layer). The
basal
cells form strong cell-to-cell junctions called desmosomes not only with
adjacent cells, but also with
the
basal lamina between
themselves
and the underlying connective tissue. During development, some areas of basal
cells divide at a different
rate,
forming epidermal ridges that extend down in the
dermis,
and the dermal tissue proliferates to form dermal papillae. This results in the
formation of deep ridges that get transmitted through the other layers of the
skin to form
fingerprints
on the surface. Fingerprints are unique to every individual
and are used for forensic
analysis
because the patterns do not change with the growth and aging processes. Even
identical twins with the same genes will have different fingerprints because of
this random process.
Stratum Spinosum
As the name suggests,
the
stratum spinosum is spiny in appearance due to the polyhedral
shape of
the
cells and desmosomes visible when tissue is prepared for
microscope slides. As basal cells divide at different rates, keratinocytes get
pushed up but maintain these strong cell-to-cell connections, changing cell
shapes and forming a protective barrier. This
stratum
is composed of
eight
to 10 layers of keratinocytes, formed as a result of cell division in the
stratum basale. Interspersed among the keratinocytes of this layer are the
Langerhans
cells,
which help with immunity.
Stratum Granulosum
The
stratum
granulosum has a grainy appearance due to further changes to the
keratinocytes as they move up from
the
stratum spinosum. The cells
(three
to
five
layers deep) become flatter, and their cell membranes thicken. At this point,
the keratinocytes generate large amounts of the proteins keratin and
keratohyalin in the cytoplasm
and,
with other lipids and enzymes, form vesicles called lamellar granules,
which
may be secreted by exocytosis. The cellular secretions act to retard water loss
and entry of foreign materials. These two proteins eventually make up the entire
mass of the keratinocytes in the stratum granulosum (the nuclei and other cell
organelles disintegrate) and mark the transition between
the
metabolically active strata and the dead
cells
of the superficial strata.
Stratum Lucidum
The stratum
lucidum
appears lucid, or clear, and is not present throughout the
body, but only on parts with
thick
skin, such as the surface of
the
palms and
the
soles of the feet. The stratum lucidum is a smooth, clear,
thin layer, just superficial to the stratum granulosum. The keratinocytes in
this layer are derived from the stratum granulosum, and mainly consist of
keratin fibers. They are flat and densely packed.
Stratum Corneum
The stratum
corneum
is the most superficial layer of the epidermis, and is the
layer that is exposed to the environment. The increased keratinization (also
called
"cornification")
of the cells in this layer gives it its name. There are usually 15 to 30 layers
of dead cells in the stratum corneum. This dry, dead layer prevents the growth
of microbes and keeps the rest of the underlying layers healthy. It is also
resistant to penetration
by
water and protects the inner layers from environmental damage. Dead cells in
this layer are shed periodically (approximately every
two
weeks) and are replaced by cells from
the
stratum granulosum (or stratum lucidum in the case of
the
palms and soles).
Example
Exfoliation is the removal of the outermost
layer of dead cells. Cosmetic procedures like microdermabrasion or chemical
peels help remove some of the dry upper layer
of
the skin and aim to keep the skin looking “fresh” and
healthy. The ancient Egyptians are credited with first discovering the
beauty effects of exfoliation. Too much exfoliation can cause damage to
underlying, living tissue.
The thickness of thick skin is a function of the four upper layers of the epidermis:
the
stratum spinosum, stratum granulosum, stratum lucidum, and stratum
corneum. The stratum corneum, consisting of keratin-packed dead cells, is substantially
thicker in thick skin than in thin skin
(more
than 300 layers versus 15 layers of cells). The palms of your hands,
soles of your feet, and your lips have thick skin. Thick skin is adapted to specialized
activities, including gripping, walking and suckling, and the wear and tear that goes
with
those
activities. Thick skin does not have hair, and has few glands.
Most of the rest of your skin is thin. The stratum lucidum isn’t even present in thin skin.
The packed keratin provides most of the protective properties associated with the
epidermis. Whereas the stratum corneum of thin skin may be completely shed and replaced
in about a
week,
this replacement may take about a month in thick skin.
Example
Calluses
When you wear shoes that do not fit well and are a constant source of abrasion on your toes,
you tend to form calluses
at the point of contact. This occurs because the basal stem cells
in the stratum basale are triggered to divide more often. This increases the
thickness of the skin at the point of abrasion so as to provide greater protection
to the underlying tissue. Calluses can also form on your fingers if they are subject
to constant mechanical stress like long periods of
writing
or
playing
stringed
instruments
or video games. The formation of calluses is an example of how tissues can adapt to
a minor or local stress. Corns
are a specialized form of calluses. They are formed due to the
abrasion on the skin as a result of an elliptical-type motion.
The skin's dermis is made up of two distinct layers of connective tissue. The
papillary layer is made up of areolar connective tissue
and the underlying reticular layer is composed of
dense
irregular connective tissue. This dermal part of the skin (organ) is vasculated (has
blood vessels) and is innervated (has nerves). As described earlier, the dermis is
sparsely populated with fibroblasts that produce collagen and elastin fibers in the
extracellular matrix. This leads to a strong and elastic tissue structure. The matrix
can also contain mast cells involved in allergic reactions.
Papillary Layer
The fibroblasts are dispersed within the collagen and elastin fibers of the areolar tissue
(loose connective tissue) of the papillary layer. This forms a loose mat, which
contains
an abundance of small blood vessels. The dermal papillae with blood capillaries
interdigitate
(become
interlocked) with the epidermal ridges of the stratum basale.
In addition, the papillary layer contains
phagocytes
— defensive cells that help fight bacteria or other infections
that have breached the skin. This layer is also interspersed with lymph vessels
and sensory receptors.
Reticular Layer
The reticular layer appears “reticulated” (net-like)
because
it is composed of
a
mesh of collagen fibers and elastin fibers. Fibrocytes form the bundles of
collagen that extend into the papillary layer and the hypodermis, making these
layers hard to distinguish. The flexible collagen provides structure and
strength, while elastin lends limited elasticity to the skin. Collagen also
binds with water, keeping the skin hydrated. Water is necessary to maintain the
normal elasticity and resiliency (called
"turgor")
of the skin. Dehydration causes a loss of turgor;
if
the skin
of a
dehydrated person is pinched it remains
domed
and does not immediately flatten out.
Collagen injections and Retin-A creams help restore skin turgor by introducing
collagen externally in the former case or by stimulating blood flow and repair
of the dermis in the latter case.
The hypodermis (also called the subcutis or subcutaneous layer)
functions to connect the integument (epidermis and dermis) to the underlying muscles and
organs. The hypodermis is not considered part of the
skin,
but has several important functions. Like the dermis, the hypodermis is made up of
areolar tissue, collagen, and elastic
fibers,
providing it
with
some elasticity. Additionally, it contains adipose
tissue, which functions as a mode of fat storage. The hypodermis is vascular and
contains arteries, veins, and blood capillaries.
Adipose tissue present in the hypodermis
accumulates
fat, which serves as an energy reserve, insulates the body, and prevents heat loss. The
fat distribution changes as our bodies mature and age.
It
is also hormone-dependent. Men tend to accumulate fat in different areas (neck, arms,
lower back, and abdomen) than do women (breasts, hips, thighs, and
buttocks). Physical inactivity due to a lack of exercise and
sedentary
jobs,
combined with the consumption of
high-calorie
foods,
has resulted in the highest rates of obesity ever seen in our country. While
accumulation of fat provided an evolutionary advantage to our ancestors, who experienced
unpredictable bouts of famine, it is now considered a major health threat. Improved diet
and increased exercise are the best ways to control body fat accumulation, especially
when it gets to levels that increase the risk of heart disease.
Accessory structures of the skin include hair, nails, sweat glands, and sebaceous glands.
Although these structures appear to be part of the dermis,
they
are actually derived from the epidermis. The hair shaft is made of dead, keratinized
cells and gets its color from melanin pigments. Nails are also keratinized and protect
the extremities of our fingers and toes from mechanical damage. Sweat glands and
sebaceous glands produce sweat and sebum, respectively. Each of these fluids has a role
to play in maintaining homeostasis. Sweat helps the body remove excess fluids and
electrolyte wastes and also cools down the body surface when it gets overheated. Sebum
acts as a natural moisturizer of the dead, flaky outer keratin layer of skin and hair.
Sebum is also known for its microbicidal and
microbiostatic
properties.
Hair
is part of the integumentary system. Strands of hair originate from
the base of the downward extension of living epithelial cells into the dermis
that is
called the hair follicle.
Hair
follicles are surrounded by the dermis, but the cells are part of the epidermis and
are
separated from the dermis by
basal
lamina layer. Hair forms in a manner similar to the skin: rapid
division and differentiation of stem cells into keratinocytes that get pushed up and
become flattened, dead, keratinized cells. The part of hair that is exposed on the skin
surface is called the hair shaft, and the rest of the follicle is called
the hair root. The bulge at the base of the hair root is called the
hair bulb, which is made up of a layer of basal cells called the
hair matrix. The hair matrix contains the cells that rapidly divide to
form the hair. The hair bulb surrounds the hair papilla (made up of
connective tissue, blood capillaries and nerve endings).
Just as the layers of the skin form on the inner layers and get pushed out to the surface
as the dead skin on the surface sheds, basal cells in the center of the hair bulb divide
to form layers of keratinocytes that form the medulla, cortex,
and cuticle of the hair bulb. These layers are visible in a longitudinal
section of the hair follicle. Keratin formation starts in the cells of the medulla and the keratin
continues to be produced in the cortex and cuticle. Keratinization is completed as the cells
are pushed to the skin surface to form the shaft of hair that is externally visible. The
external hair is composed entirely of keratin.
Additionally, the hair follicle is made up of three concentric layers that make up the wall of
the follicle
—
the internal root sheath, the external root sheath, and the
glassy membrane. The cells of the internal root sheath are derived from
the basal cells of the hair matrix. This layer does not surround the entire hair strand,
but stops short at the base of the hair shaft. The external root sheath, which encloses
the hair root, is made up of basal cells at the base of the hair root and tends to be
more keratinous in the upper regions. The glassy membrane is a thick, clear connective
tissue sheath covering the hair root and connecting it to the tissue of the dermis.
Hair serves a variety of functions. For example, hair on the head protects it from the sun and
from
heat loss; and hair in the nose and ears and around the eyes
(eyelashes)
defends
the body by trapping dust particles that may contain allergens and microbes. Hair on the
eyebrows prevents sweat and other particles from bothering the eyes. Hair also has a
sensory function due to innervation of the hair papilla. Hair is extremely sensitive to
changes in the environment, much more so than the skin surface. The hair root is
connected to smooth muscles called arrector pili that contract in response
to stimuli, making the external hair shaft “stand up.” This is visible in humans as
goose bumps and even more obvious in animals, such as when a frightened cat’s fur puffs
out. In many
animals,
the puffing out
makes the
animal
appear
larger,
and could
possibly enable it
to
scare off a predator. Another advantage of this ability to cause the hair to stand up is
that it can trap air and can act as an
insulator,
decreasing heat loss.
Hairs grow during a
phase
called
anagen,
and they
are eventually shed, only to be replaced by newer ones. When hair is
naturally ready to be shed, the follicle becomes inactive during a phase called catagen.
The follicle then becomes smaller, and becomes detached from the dermal papilla at the
base,
during the phase called telogen. The basal cells in the hair matrix then produce a new
hair follicle during anagen. Hair typically grows at the rate of 0.3
mm per day and can continue growing for
two
to
five
years
before being shed. About 50 hairs may be lost and replaced per day. Hair loss occurs if
there is more hair shed than what is
replaced,
and it
can happen due to hormonal
or
dietary changes.

Just like the skin, hair gets its color from the pigment melanin, produced by melanocytes
in the hair matrix. Different hair color results from differences in the type of
melanin, which is genetically determined. As a person ages, the melanin production
decreases and hair tends to lose its color, becoming gray and/or white.
Example
Alopecia
Many individuals can experience hair thinning and/or loss with advanced age. This is called
alopecia. This can lead to complete hair
loss,
called baldness. Most baldness is caused by a genetic sensitivity of hair follicles
to the androgen hormone dihydrotestosterone (DHT). DHT causes the hair follicle to
receive less blood
flow,
so that the hair follicle begins to atrophy and any hair that is produced is
thin.
A common form of baldness is male pattern
baldness,
which results from a mutation on the X chromosome. It is seen more often in men
because men only receive one copy of the X chromosome. Many women may be carriers of
this trait, having one normal X
chromosome
and one X
chromosome
that has the male pattern baldness gene.
Women
usually do not exhibit the balding patterns seen in men.
The nail is a specialized structure of the epidermis that occurs at the tips of our fingers and
toes. The nail body is formed on the nail bed, and it is
designed to protect the tips of our fingers and toes, as they are the farthest
extremities and the parts of the body that experience the maximum mechanical stress. The
epidermis in this part of the body has evolved a specialized structure upon which nails
can form. The nail body forms at the nail root. Lateral nail
folds, folds of skin that overlap the nail on its side, help anchor the nail
body. The nail fold that meets the proximal end of the nail body forms the nail
cuticle, also called the eponychium. The nail bed is rich in blood
vessels,
making it appear pink, except at the base, where there is a
crescent-shaped
region called the lunula. The nail body is composed of keratin-rich,
densely packed dead keratinocytes. The area beneath the free edge of the nail, where
debris gets lodged, is called the hyponychium.
Sweat (Sudoriferous) Glands
When the body becomes overheated, sweat is produced to cool the body's temperature and
prevent overheating. There are two types of sweat glands responsible for
excreting sweat —
eccrine
(merocrine)
sweat glands and apocrine sweat glands. Eccrine
glands are present throughout the skin surface, especially on the palms of the
hand, the soles of the feet, and the forehead. Like hair and
nails,
they are derived from the epidermis. They are coiled glands that lie in the
dermis, with the duct opening to a pore on the skin surface, where the sweat is released
(although
some may open into hair
follicles,
like sebaceous
glands).
The sweat released by eccrine sweat glands is mostly water, with some salt,
antibodies, traces of metabolic waste, and a microbe-killing compound called
dermcidin.
The main function of eccrine sweat glands is to help regulate body temperature
through evaporation.
Eccrine glands are controlled by the sympathetic division of the autonomic nervous system.
The sympathetic division is known as the "fight or flight" division. When you
are
nervous,
you might notice that your palms sweat. This is because when the sympathetic
division is
activated,
it triggers sweating.
Apocrine glands are usually associated with hair follicles and are activated in densely hairy
areas like armpits and genitals. They are larger than merocrine sweat glands and
lie deeper in the dermis, sometimes even reaching the hypodermis. They release a
thicker fluid due to a higher concentration of fatty
acids,
which may give it a whitish color. These fats are often decomposed by bacteria
on the skin, resulting in an unpleasant odor, commonly called body odor.
Apocrine glands do not begin to function until puberty. Apocrine sweat glands
are stimulated during emotional stress and sexual excitement.
Sebaceous Glands
Sebaceous glands are oil glands that are found all over the body. Most are
associated with hair follicles. They generate and excrete a mixture of lipids,
called sebum, onto the hair and skin surface, thereby naturally lubricating the
dry and dead layer of keratinized cells of the stratum corneum and hair shaft.
Sebum also has antibacterial properties, and prevents water loss from the skin
in low-humidity environments. The secretion of sebum is stimulated by hormones,
many of which do not become active until puberty. Thus, sebaceous glands are
relatively inactive during childhood and become active only after puberty has
occurred.
Example
Acne
Acne is a skin disturbance that typically occurs on areas of the skin
that are rich in sebaceous glands
(the
face and back). It is most common
during
the onset of puberty due to associated hormonal changes, but can continue
into adulthood.
Hormones
such as androgen and other sex steroid
hormones
stimulate the release of sebum. When sebaceous glands overproduce and get
blocked with sebum, it leads to the formation of
blackheads. Blackheads are the result of
hyperkeratinization of the area,
which
causes the formation of a keratin
plug
and blockage of hair follicles in the area. Blackheads are prone to
infection by acne-causing bacteria (i.e.
Propionibacterium
and Staphylococcus), which leads to redness and swelling. In severe
cases, acne can lead to scarring due to the production of scar tissue
during
the wound healing process.
In earlier sections,
you learned
about
the amazing regenerative capacity of skin: Basal cells divide and differentiate to form
keratinocytes,
which move superficially to the surface and change their structure along the way. There
are some cases where this regenerative property can cause
problems,
and some traumas where regeneration is put to the test.
Example
Psoriasis
Psoriasis is an
autoimmune
disorder where too many skin cells are produced. Skin rapidly accumulates and looks
silvery-white in appearance. Plaques from plaque psoriasis frequently occur on the
skin of the elbows and knees, but can affect any area, including the scalp, palms of
hands and soles of feet, and genitals. The disorder is a chronic recurring condition
that varies in severity from minor localized patches to complete body coverage.
Wound Repair and Scarring
The first step to repairing damaged skin is the formation of a blood clot
that
scabs over with time. Many different types of cells are involved in wound
repair, especially if the surface area that needs repair is extensive. Before
the basal stem cells of stratum basale can
re-create
the epidermis, fibroblasts and mesenchymal cells mobilize and divide rapidly to
repair the damaged tissue, forming a loose, highly vascular tissue called
granulation tissue. This increased vascular network helps deliver the oxygen and
nutrients necessary for further repair. Immune cells, like macrophages, roam the
area and engulf any foreign
matter,
reducing the chance of infection while also clearing away any tissue debris.
Scars occur when there is repair of skin damage, but the healing process
prevents regeneration of the original skin structure. Almost every cut or wound,
with the exception of ones that only scratch the surface (epidermal wounds),
leads to some degree of scar formation. As the tissue tries to repair itself to
the original state, fibroblasts often generate a greater density of collagen
fibers than were in the original tissue, which is what results in scar
formation. This dense, fibrous structure (connective tissue) has few cells and
does not allow for the regeneration of accessory structures (epithelial tissue)
like hair follicles, sweat
glands,
or sebaceous glands.
Sometimes, scarring does not stop when the wound is healed. This results in the
formation of a raised or hypertrophic scar called a keloid. In
contrast, scars that result from acne and chickenpox have a sunken appearance
and are called atrophic scars.
Scarring of skin after wound healing is a natural process and does not need to be
treated further. Application of oil and lotions may reduce the formation of scar
tissue by keeping the skin soft and pliable as it heals, allowing the separate
edges to be pulled together. However, modern cosmetic procedures like
dermabrasion, laser treatments, and filler injections have been invented as
remedies for severe scarring. All of these procedures try to reorganize the
structure of the epidermis and underlying collagen in the connective tissue to
make it look more natural.
Burns are wounds that result when the skin is exposed to intense heat, radiation,
electrical current, or caustic chemicals. Burns cause skin cells to die, disrupting the
epithelial barrier that normally prevents fluid loss. Depending on the severity of the
burn, dehydration, electrolyte imbalance, and renal and circulatory
failure
may follow. Burn patients are treated with intravenous fluids to offset the dehydration,
as well as intravenous nutrients that enable the body to repair tissues and replenish
lost proteins. Another serious threat to a burn patient is the high possibility of
infection. Burned skin is extremely susceptible to bacteria and other pathogens, as the
loss of body covering provides a direct pathway for the entry of microorganisms.
Burns are classified by the degree of their severity. In first-degree burns, only
the epidermis is damaged. Although painful, these burns typically heal on their own
within a few days. A typical sunburn is a first-degree burn. Second-degree
burns destroy both the epidermis and the dermis. They result in painful
blistering of the skin. It is important for individuals with second-degree burns to keep
the area clean and sterile in order to prevent infection. If this is done, the burns
heal on their own within several weeks.
Third-degree burns are more serious and penetrate the full thickness of the skin,
including the subcutaneous (hypodermis) layer. The skin may appear white, red, or black.
Fourth-degree burns are the most severe, affecting the underlying
muscle and bone as well.
Third-
and
fourth-degree
burns are usually not as painful because the nerve endings themselves are damaged.
However, a
third- or
fourth-degree
burn results in rapid water loss and infection due to the exposure of the underlying
tissue, and requires emergency trauma care. Full-thickness burns cannot be repaired
locally because the local repair machinery itself is
damaged.
Full-thickness
burns
require excision of the affected tissue (amputation in severe cases), followed by
grafting of skin from an unaffected part of the body, or from skin grown in tissue
culture for grafting purposes.
Puberty
During puberty, the changes in the endocrine system produce hormones that
regulate aspects of the integumentary system. Sebaceous glands, apocrine sweat
glands and hair growth become active or more active.
Advanced
Aging
All organ systems in the body, including the integumentary system, undergo subtle changes
over time that add up as a person ages. Reductions
in
metabolic
activity
and
cell division, lowered blood circulation, and decreased hormonal levels are some
of the basic changes that occur in the human body as it ages.
The epidermis is made up of several layers of cells that are shed and replaced by
basal
stem cell division. With aging, these cells divide less reliably and aging skin
becomes thinner. The dermis, which is responsible for the elasticity and
resilience of the skin, also weakens since elastin fibers become
cross-linked
and don’t stretch. Repair of wounds occurs more slowly as a person ages. The
hypodermis, which stores fats in its adipose tissue, loses its structure due to
the reduction and redistribution of fat, which in turn is affected by hormonal
levels. The accessory structures also have lowered activity, generating thinner
hair and nails, and reduced amounts of sebum and sweat. Reduced sweating ability
causes the elderly to be unable to tolerate extreme heat. Other cells in the
skin,
such
as
melanocytes and dendritic cells, also become less active, leading to a paler
appearance and lowered immunity. Wrinkling of skin occurs mainly due to
the
breakdown of
collagen
and elastin,
the
weakening of muscles under the skin, and the inability of the
skin to retain adequate moisture.
Many anti-aging products line the shelves of stores today. These products (mainly composed of
tretinoin,
or
Retin-A)
try to introduce more structure into the skin, first by
rehydrating
the skin with moisturizers, and then
by
triggering mitotic activity of the underlying cells to
encourage tissue formation and regeneration. Some products contain epidermal
growth factor, which
induces
collagen synthesis, the end-product of wound
healing,
to give the skin a healthier look.
The integumentary system regulates body temperature through several different means. Recall
that sweat glands
—
accessory structures to the
skin
— excrete water, salt, and other substances to cool the body when it
becomes warm. This is termed
"sensible
perspiration."
Even if the body does not appear to be noticeably sweating, approximately 500 mL of
sweat are secreted a day. If the body becomes excessively warm due to high temperatures,
vigorous activity, or a combination of the two, the blood vessels in the integumentary
system dilate and sweat glands produce large amounts of sweat
—
up to
three
gallons a day. As the sweat evaporates from the skin surface, the body is
cooled;
in a
way,
the skin surface acts like the radiator of a car. The dilated blood vessels in the
dermis
that account for the redness that many people experience when exercising on a hot
day
also help in the dissipation of body heat by increasing the superficial blood flow.
When temperatures are cold, the adipose tissue of the hypodermis helps insulate the body.
Goosebumps
and the arrector pili muscles help to insulate by trapping air on the surface of the
skin. Additionally, the dermal blood vessels constrict to decrease blood flow (and heat
loss) at the skin.
The fact that you can feel an ant crawling on your skin, causing you to flick it off
before it bites, is due to the fact that the sensory receptors in the skin (especially
the hair root plexus associated with the hair in the follicles) can sense changes in the
environment. This occurs via sensory receptors that convert physical or chemical stimuli
into electrical signals that are sent to the central nervous system (CNS; brain and
spinal cord). The CNS then processes this sensory input and generates a voluntary
response to the ant. The skin acts as a sense organ because the dermis and
hypodermis contain sensory receptors that extend into the epidermis. These
receptors, such as Merkel discs, are more concentrated at the fingertips and lips, which
are most sensitive to touch. Examples of other sensory receptors present in the skin are
tactile
(Meissner's)
corpuscles and lamellated (Pacinian) corpuscles that respond to
pressure and vibration, respectively, and free nerve endings that are sensitive to
temperature (hot or cold) and pain.
Skin Conditions and General Health
There are a variety of conditions that can change the appearance of the skin. In
vitiligo, the melanocytes in certain areas lose the ability to produce melanin,
possibly due to an autoimmune reaction, leading to loss of pigmentation in
patches. Liver disease or liver cancer can cause an accumulation of bile and the
yellow pigment bilirubin, leading to the skin appearing yellow or jaundiced
("jaune" is French for "yellow"). Tumors of the pituitary gland can result in
the secretion of large amounts of
corticotropin-releasing
hormone (CRH), which can stimulate excessive levels of melanocyte-stimulating
hormone (MSH) and adrenocorticotropic hormone (ACTH). In Addison’s disease
excessive CRH stimulates production of adrenocorticotropic hormone (ACTH) and
MSH. In these diseases, the skin takes on a deep bronze color.
The oxygen content of blood in the dermal vascular system is reflected in skin
hue. Healthy individuals who have adequate supplies of the red-colored
oxygen-bound hemoglobin usually have a pink hue to their cheeks and lips.
Indeed, hemoglobin and the vasculature of the skin also contributes to skin
color in people with fairer skin. A sudden drop in oxygenation of the skin can
initially cause the skin to turn ashen (or white). If there is a prolonged
reduction in oxygen levels, it makes the skin appear blue (cyanosis), which
happens when a person has a limited oxygen supply to the body. The blue color is
most obvious in areas where blood capillaries are close to the surface, like the
lips and nail beds. Blue lips is a sign of hypoxia and hypothermia, which lead
to drastically reduced peripheral circulation, or of any other condition that
prevents adequate oxygenation of the blood, which can occur in a variety of lung
diseases. At the other extreme, elevated blood levels of carbon monoxide can
cause the lips and oral mucosa to appear bright cherry red because the carbon
monoxide molecule binds to hemoglobin tighter than oxygen, giving the hemoglobin
in the red blood cells the bright red color. Carbon monoxide poisoning is fatal,
and prompt treatment with pure oxygen is indicated.
Maintaining homeostasis within the body requires the coordination of many
different systems and organs. Communication between neighboring cells, and
between cells and tissues in distant parts of the
body,
is done through either the nervous system or the endocrine system. The nervous
system utilizes electrochemical impulses to regulate muscles and glands. These
signals, carried by neurons, elicit responses in target cells within
milliseconds. However, the duration of
those
responses
is very brief unless neuronal signalling continues. The endocrine
system regulates biological processes through the release of
chemicals called hormones. Hormones are released into body
fluids—usually
blood, which carries these chemicals to their target
cells,
where they elicit a response. The responses elicited by hormones usually take
several seconds to several days to occur,
and
the duration of the response can last just as long without further signalling.
Unlike nearly instantaneous nerve impulses, hormones of the endocrine system can
regulate functions of the body on longer time scales to maintain
homeostasis.
The major function of the endocrine system is to participate in homeostatic feedback
loops by acting as a means of communication between integrators and effectors, and
sometimes acting as the sensor as well. Since the function of the endocrine system is to
maintain homeostasis, there will be many system level examples at the end of this
chapter. Also, this is an excellent time to review the concept and formal structure of
homeostasis if you are uncertain or out of practice.
The endocrine system is also involved with growth, development and adaptation. Within all
of these changing processes, our bodies tolerate fluctuations within certain limits but
still overall homeostasis needs to be maintained.
Example
Endocrine System Homeostasis and Integration with Other Systems
When we grow in height, our bones increase in length. However, proper homeostasis
must be maintained to ensure that we always have calcified bone to support our
weight. Muscles, tendons and ligaments must also grow proportionately. The endocrine
system integrates these changes while still maintaining homeostasis.
Common Dysfunctions of the Endocrine System
We feel the effects of changes in the endocrine system at various points in our
lives. Hormone levels fluctuate during puberty, and even after we eat a meal.
Common dysfunctions of the endocrine system include an inability to regulate
glucose, called diabetes mellitus, and an inability to regulate calcium levels
in the bones,
which
may lead to osteoporosis. Other common disorders of the endocrine system include
over- or
under-production
of thyroid hormone (hyperthyroidism and hypothyroidism, respectively), which
impacts energy metabolism. In this unit,
you
will learn
about
dysfunctions of the endocrine system and the downstream results of such
dysfunctions.
The lipid-derived hormones include steroid hormones and eicosanoids.
Steroid hormones, the primary class of lipid hormones in humans, are
derived from cholesterol and show structural
similarity
to that molecule. Examples of steroid hormones include estrogen and
testosterone, which are released by reproductive organs, and aldosterone and cortisol,
which are released by the adrenal glands.
Steroid
hormones
are released as synthesized. They are insoluble in water, so to
circulate through the blood they bind to transport proteins in serum. Since they are
bound to carrier proteins, steroid hormones typically remain in circulation longer than
other classes of hormones.
Recall
that the "lifetime" of a molecule from production to destruction is called the
half-life. The steroid hormone cortisol has a half-life of
60 to
90
minutes, whereas epinephrine, an amino acid-derived hormone, has a half-life of
approximately one minute. They are eventually degraded and excreted in urine or
bile.
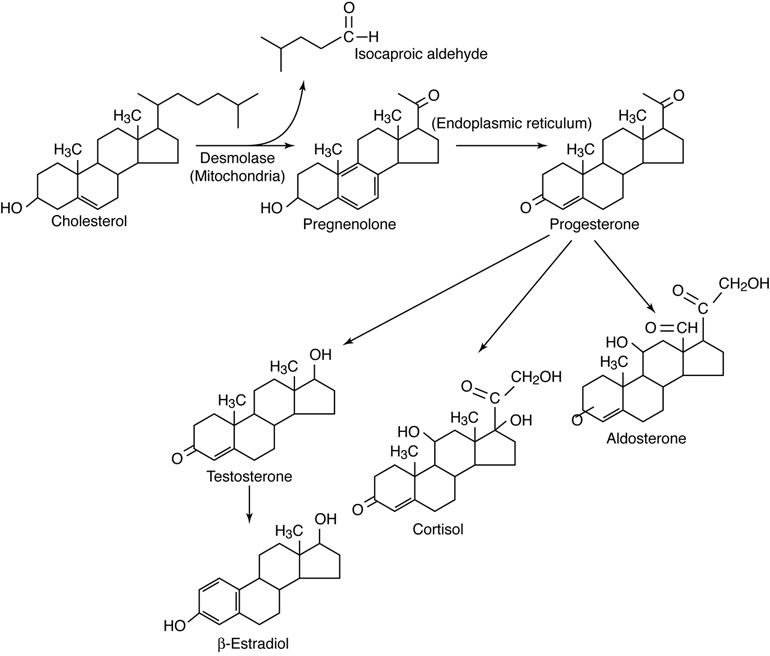 Biosynthetic pathways for some selected steroid hormones.
Biosynthetic pathways for some selected steroid hormones.
Eicosanoids
are a class of signalling molecules derived from polyunsaturated fatty acids. They are
typically released as
paracrine
signals upon stimulation and are only active for a few seconds.
Of the amino acid-derived hormones, some circulate for only a few minutes
while others may circulate for days. The amino acid tyrosine is the precursor for two
groups of hormones: the thyroid hormones, produced in the thyroid gland; and the
catecholamines (epinephrine and norepinephrine), produced in the adrenal medullae. The
amino acid tryptophan is the precursor for the hormone melatonin, secreted by the pineal
gland, and the hormone serotonin, which is quite widespread in the body. Some of these
hormones have
a
circulating half-life of a few days (thyroid hormones) while some are
rapidly degraded (catecholamines).
Peptide hormones are short peptides and
polypeptide
chains.
Recall
that peptides are usually
made up of
fewer than 50 amino acids and proteins
are
typically longer than that. Some peptide hormones of longer lengths
can have secondary
structures.
Protein hormones, being much longer than peptides, can have more extensive
three-dimensional structures and can even be globular.
Peptide hormones are a diverse group and include molecules that are only a few amino
acids, such as antidiuretic hormone and oxytocin (each with 9 amino acids), produced by
the pituitary gland. This class also includes proteins, such as growth hormone (191
amino acids), and glycoproteins, such as follicle-stimulating hormone (92 amino acids
with glycosylation). Peptide hormones can either be released as produced or stored and
released in response to stimulus. Most are water soluble and are easily transported in
blood plasma, but some also bind to transport proteins. Most have a half life of
minutes.
Hormone production can be regulated by positive and negative feedback pathways. In positive feedback systems, the
release of a hormone leads to an action that stimulates release of more of the same
hormone.
Example
Positive Feedback Loop in the Endocrine System
Oxytocin released by the pituitary gland prior to childbirth stimulates contraction
of the uterus and increases pressure on the cervix. The increased pressure signals
the pituitary to release even more oxytocin, which increases force of contractions,
leading to even more cervical pressure. This amplification cycle continues until
childbirth is complete.
In a hormonal negative feedback loop, when a stimulus causes the release of a hormone
(Hormone A), the hormone binds to the target cell receptor, causing the necessary
metabolic change toward homeostasis. In a negative loop, the effect of this metabolic
change is to counter the stimulus that caused the release of Hormone A. Once the cause
of the stimulus returns to normal range, the production of that hormone stops and plasma
level of that hormone returns to the normal (pre-stimulus) level. In this way, the
concentration of most hormones in blood is maintained within a narrow range.
Example
Negative Feedback Loop in the Endocrine System
The hypothalamus monitors the plasma level of thyroid hormones, among others. When
the level drops, the hypothalamus stimulates the anterior pituitary to release a
hormone (Thyroid Stimulating Hormone, or Thyrotropin) that stimulates the thyroid to
release thyroid hormones. Increased levels of the thyroid hormones in the blood then
feed back to the hypothalamus and anterior pituitary to inhibit further stimulation
of the thyroid gland. Other homeostatic imbalances, such as low body temperature,
can also stimulate the hypothalamus to stimulate the anterior pituitary to release
thyrotropin. Thyroid hormones play an important role in metabolic heat production
via ATP production.
There are three mechanisms by which endocrine glands are stimulated to synthesize and
release hormones: humoral regulation, hormonal regulation, and
neural regulation.
Humoral Stimuli
The term humoral is derived from the term humor, which refers to bodily fluids such as blood and
other extracellular fluids. Humoral stimuli regulate the release of
hormones in response to specific changes in extracellular fluids, such as the
concentration of a particular ion or solute in the blood or even the overall
solute levels in the blood.
Example
A rise in blood glucose level triggers the pancreatic release of insulin.
Insulin causes blood glucose levels to drop, which signals the pancreas to
decrease insulin production through a negative feedback loop. Similarly, low
blood calcium stimulates the release of parathyroid hormone from the
parathyroid gland which stimulates the release of calcium from bone,
decreases calcium excretion in urine and promotes calcium absorption in the
digestive system.
Tropic Hormonal Stimuli
With tropic hormonal stimuli, a hormone is produced and released by
an endocrine gland in response to another hormone (known as "tropic hormones").
These hormones controlling release of another hormone are called
tropic (meaning "turn toward," pronounced “tro’-pick”; not same
as geographical “trop-ic”).
Example
The hypothalamus produces hormones that stimulate the anterior pituitary. The
anterior pituitary in turn releases hormones that regulate hormone
production by other endocrine glands. For example, the anterior pituitary
releases thyroid-stimulating hormone, which stimulates the thyroid gland to
produce the hormones T3 and T4. As blood
concentrations of T3 and T4 rise, they inhibit further
hormone production by both the pituitary and the hypothalamus in a negative
feedback loop.
Note...Sometimes students get confused between tropic and trophic hormones.
Tropic hormones stimulate release of other hormones from
endocrine cells, such as those discussed here. Trophic (meaning
“nourishment or nurse”) horomones stimulate non-endocrine cell growth and
development, such as growth hormone, estrogen and testosterone.
Neural Stimuli
The nervous system can also directly stimulate endocrine glands to release
hormones through a mechanism known as neural stimuli.
Example
Neuronal signaling from the sympathetic nervous system directly stimulates
the adrenal medulla to release the hormones epinephrine and norepinephrine
in response to stress.
Length Scale of Hormonal Signaling
Cells communicate with one another via chemical messengers. The communication may
happen between cells close by or far away from the cells that produces the
messenger (signal). For example, released hormones travel throughout the body
and affect any cells with receptors for the specific hormones. Autocrine
signaling (auto- means self) affects the
cells that released the signaling molecule. Autocrine signaling
(auto- means self) affects local cells other than
the secreting cells. While traditionally a hormone is thought to have its effect
at a distances from where it is secreted, the definition of hormones now include
paracrine and autocrine mechanisms as well. The all-inclusive term, Endocrine
signaling, includes all types of communication where chemical molecules produced
from a cell affect the metabolism of another cell (paracrine or endocrine) or
that of its own (autocrine).
Steroid hormones are lipophilic and need transport proteins in the blood. Once released
from their transport protein, the non-polar hormone is able to diffuse across the plasma
membrane of cells. Recall that the lipid bilayer of the plasma membrane of cells uses
amphiphilic phospholipids to compartmentalize the cytoplasm of a cell. When a steroid
hormone crosses the plasma membrane of a target cell, it binds to an intracellular
hormone receptor in the cytoplasm, on intracellular membrane system (ER) or,
within the nucleus of the cell. The receptor/hormone complex can then bind to a specific
site on DNA and act as a transcription regulator to increase or decrease the synthesis
of particular mRNA molecules coded by these specific genes. This, in turn, alters mRNA
production, which determines the amount of corresponding protein that is synthesized.
The steroid hormone regulates specific cell processes. The rate of transcription and
protein synthesis is directly proportional to the amount of hormone forming
receptor/hormone complexes; so if the hormone production increases, so does the
physiological effect in the body.
The thyroid hormones, T3 and T4, also use plasma transport proteins
and intracellular DNA-binding receptors. There are, however, some important
physiological differences with the steroid hormone mechanism. The target cells have
membrane transport proteins that transport the thyroid hormones into the cell. The
Thyroid hormone receptor is found bound to a transcription repressor protein on the DNA.
The binding of the hormone with the receptor-repressor complex to form the
receptor/hormone complex causes the repressor protein to dissociate, and a transctiption
activator protein becomes associated with the receptor. This, in turn, initiates
transcription.
Most peptide and amino acid hormones are polar and therefore cannot diffuse through the
plasma membrane of cells. So, they bind to plasma membrane hormone
receptors on the outer surface of the plasma membrane. Unlike steroid
hormones, polar hormones also alter intracellular processes and can also affect the
target cell’s transcription. Since they cannot enter the cell and act directly on any
DNA-binding proteins, they exert their transcriptional effects through intermediate
molecules called second messengers as described below. Catecholamines (amine class) and
polar eicosanoids (lipid-derived class) also bind to cell-surface hormone receptors.
Binding of these hormones to a cell membrane surface receptor results in activation of a
signaling pathway that triggers a cascade of intracellular activity and specific effects
associated with the hormone. Most hormones that bind at the surface receptor remain
outside the target cell. Some hormones are taken into the cell by endocytosis to
initiate the intracellular biochemical response from within vesicles. The hormone that
initiates the signaling pathway, the first messenger, activates a second messenger
within the cell.
Example
Glucagon is produced when blood sugar drops. The glucagon binds to its receptor on
target liver cells and stimulates two different metabolic reactions inside the cell.
The liver cells will be stimulated to break down stored glycogen to glucose and to
synthesize new glucose from some amino acids. As a result, glucose is released into
the blood and blood sugar returns to normal.
G-proteins are a class of trans-membrane cell surface proteins that can be activated by
hormones or ions and other chemicals for cell signaling. G-proteins remain inactive
unless a hormone is bound to its cell surface receptor. Inactive G-proteins are bound to
GDP on its cytoplasmic side. When a hormone binds to the cell surface receptor the bound
GDP is replaced by GTP.
Activated G-proteins can have different functions depending on the hormone receptor: it
can open a membrane protein channel; it can release a small molecule; or it can activate
a membrane-bound enzyme. There is a large variety of G-protein-induced effects. For
example, G-protein-linked ion channels can stimulate movements of ions across the
membranes. There are specific channels for potassium, sodium, calcium or chloride.
For G-protein activated membrane-bound enzymes, there are large numbers of activated
enzymes. One of the membrane bound enzymes that the G-Protein coupled receptor (GPCR)
activates upon binding to a hormone molecule is the adenylate cyclase. This enzyme
catalyzes the conversion of ATP to cyclic AMP (cAMP). which, in turn, activates a class
of enzymes called protein kinases. These kinases transfer a phosphate group from ATP to
a substrate molecule in a process called phosphorylation. The phosphorylation of a
substrate molecule changes its shape, thereby activating it. This chain of reactions,
where hormone binding to the GPCR leads to the appearance of cAMP in the cytoplasm as
the second messenger which, in turn, leads to the activation of protein kinase is an
example of a “reaction cascade.” In this case, since a signal from the exterior of the
cell is transferred to the interior via the formation of a “second messenger,” the
process is called “signal transduction cascade." Other second messengers that can be
involved as a result of hormone binding to a cell membrane receptor include cyclic GMP
(derived from guanosine
triphosphate),
tyrosine kinases, inositol phospholipids, and even calcium ions. Cellular responses to
hormone binding of a cell membrane receptor include altering membrane permeability,
activating metabolic pathways, stimulating synthesis of proteins and enzymes, and
hormone release.
The binding of a hormone at a single cell membrane receptor causes the activation of many
G-proteins, which can catalyze many reactions simultaneously. Thus, the effect of a
peptide hormone is amplified as the signaling cascade progresses. A small amount of
hormone can trigger the formation of a large amount of cellular product. To stop hormone
activity, the cascading chemical reaction is interrupted; for example cAMP is
deactivated by the cytoplasmic enzyme phosphodiesterase (PDE). PDE is
always present in the cell, and it breaks down cAMP spontaneously, preventing
overproduction of cellular products. The specific response of a cell to a
lipid-insoluble hormone depends on the type of receptors that are present on the cell
membrane and the substrate molecules present in the cell cytoplasm.

The pituitary-hypothalamus axis links the nervous system with the endocrine system. The
hypothalamus is a region of the brain that is located inferior to the
thalamus. It coordinates signals from internal organs and other regions of the brain and
regulates a response from the endocrine system via the pituitary. The hypothalamus
secretes both releasing hormones that stimulate the anterior pituitary to
secrete a hormone and inhibiting hormones that inhibit the release of a
hormone from the anterior pituitary.
The pituitary gland is a pea-sized gland located at the base of the brain
attached to the hypothalamus via a stalk called the infundibulum. The
pituitary gland is primarily regulated by nerve impulses or hormones released by
neurosecretory cells of the hypothalamus. The pituitary, in turn, releases hormones that
either have a direct effect on target cells or regulate hormone production by other
endocrine glands. Those hormones that control the release of another hormone from an
endocrine gland are called tropic hormones. The pituitary has two distinct
regions: the anterior pituitary, and the posterior pituitary. The anterior pituitary
secretes seven different peptide or protein hormones: growth hormone(GH), prolactin
(PRL), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ATCH),
follicle-stimulating hormone (FSH), luteinizing hormone (LH), and melanocyte stimulating
hormone (MSH). The posterior pituitary is an extension of the brain and releases
hormones produced by the hypothalamus. The posterior pituitary releases antidiuretic
hormone (ADH) (also known as vasopressin) and oxytocin. The pituitary looks like one
gland because the anterior and posterior pituitary do not have externally visible
distinctions, but from a cell and tissue perspective, they are really two different and
distinct organs.
The anterior pituitary gland, or adenohypophysis, is surrounded by a
capillary network. This capillary network is a part of the hypophyseal portal system
that carries substances from the hypothalamus directly to the anterior pituitary.
Remember that substances such as hormones can only leave or enter the circulatory system
at capillaries, and portal systems (portal systems are also found in the digestive
system) move material from one capillary bed to another without returning it to the main
circulation. Anterior pituitary hormones then enter the capillaries and travel to the
heart and through the systemic system in the same way other hormones do.
Several anterior pituitary hormones (TSH, ACTH) are tropic hormones, because
they control the functioning of other endocrine glands. While these hormones are
produced by the anterior pituitary, their production is controlled by regulatory
hormones produced by the hypothalamus. Negative feedback mechanisms regulate how much of
these regulatory hormones is released, and how much anterior pituitary hormone is
secreted.
The posterior pituitary is significantly different in structure and function
from the anterior pituitary. As its name implies, the posterior pituitary is behind the
anterior pituitary (toward the back). It contains mostly axons of secretory neurons and
neuroglial cells; the cell bodies of these neurons are in the hypothalamus. The
posterior pituitary and the infundibulum together are referred to as the
neurohypophysis.
The posterior pituitary does not produce hormones, but stores hormones produced by the
hypothalamus and releases them into the bloodstream. The hormones antidiuretic hormone
(ADH) and oxytocin are produced by neurons in the hypothalamus and transported within
these axons along the infundibulum to the posterior pituitary. They are released into
the posterior pituitary capillaries in response to neural signaling from the
hypothalamus. These hormones are considered to be posterior pituitary hormones, even
though they are produced by the hypothalamus, because that is where they are released
into the circulatory system.

The thyroid gland possesses two lobes that are connected by the isthmus. It
is located in the neck, just below the larynx with the isthmus in front of and lobes
lateral to the trachea. It has a dark red color due to its extensive vasculature. When
the thyroid increases in size due to dysfunction, it can be felt under the skin of the
neck. The main function of the thyroid gland is the synthesis and storage of thyroid
hormones that are involved in maintaining metabolic homeostasis.
The thyroid gland is made up of many spherical thyroid follicles, which are lined with
simple cuboidal epithelium. These follicles contain a viscous fluid called
colloid that stores the glycoprotein thyroglobulin, the
precursor to the two thyroid hormones. Thyroglobulin is not normally released into
circulation unless the thyroid gland is damaged due to disease or injury. Other
endocrine cells, called parafollicular cells, are located between adjacent follicles and
produce a different hormone, calcitonin, that is involved in blood calcium
homeostasis.

Unlike most endocrine glands, the thyroid gland stores large amounts of some of the
hormones it synthesizes. Thyroglobulin is produced and secreted by follicle cells into
the lumen of follicles as a colloid. There it undergoes post-translational modification
to produce functioning thyroid hormones. Iodide molecules are added to the thyroglobulin
precursor to produce the hormones thyroxine and triiodothyronine. Thyroxine is also
known as T4 because it contains four atoms of iodine, and triiodothyronine is
also known as T3 because it contains three atoms of iodine. In developed
countries, the iodide required for hormone synthesis is obtained primarily from iodized
salt. However, seafood and plants grown in iodine rich soil at lower elevations also
provide the required iodide. Since iodine as a molecule is quite volatile, the food
grown in higher elevations (lower atmospheric pressure) lacks sufficient iodine. Iodide
ions are actively transported into the follicular lumen from the capillaries by follicle
cells. Follicle cells are stimulated to release stored T3 and T4
from the lumen into the blood capillaries by thyroid stimulating hormone (TSH), which is
produced by the anterior pituitary.
Follicle cells also begin synthesizing more T3 and T4 in response
to TSH stimulation.
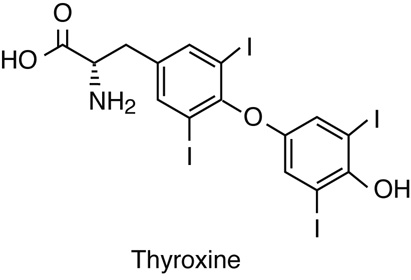
A third hormone, calcitonin, is produced by parafollicular cells, or C cells of the
thyroid. Calcitonin release is not controlled by TSH, but instead is released when
calcium ion concentrations in the blood rise. Calcium ions bind to specific receptor on
the C cell and stimulate release of calcitonin. Calcitonin acts primarily in children to
lower blood calcium levels when levels get too high. Calcitonin causes decreased tubular
reabsorption of Ca2+ in the kidneys, leading to calcium loss in urine. It
inhibits bone resorption activity of osteoclasts and calcium absorption in intestine to
also reduce plasma calcium levels. It is also suspected to have an indirect effect
stimulating osteoblast activity and development. However, in adult humans it appears to
play only a minor role in calcium homeostasis because abnormalities in calcitonin
production do not appear to be associated with specific plasma calcium imbalances.
Research has implicated its role during times of high calcium demands, such as pregnancy
and lactation.
The four parathyroid glands are each the size of a grain of rice and are
usually located on the posterior surface of the thyroid gland. The exact location and
number of parathyroid glands can vary from person to person. The parathyroid glands are
named for their proximity to the thyroid gland (para- means next to), often seeming to
be part of the same gland; however, their cells are distinct from those of the thyroid
gland.
 Note that this image is on a different anatomical plane than the previous image
of the thyroid.
Note that this image is on a different anatomical plane than the previous image
of the thyroid.
Each parathyroid gland is covered by connective tissue and contains many secretory cells
called chief cells, which synthesize and secrete parathyroid hormone (PTH).
Another type of cell, oxyphil cells, can be distinguished histologically in the
parathyroid but they are not clearly understood. They appear to increase during renal
failure, but their function is still a subject of clinical research.
PTH is synthesized as a pro-peptide that is cleaved into an active hormone, which is
vital in maintaining blood calcium levels. Calcium is required for nerve impulse
transmission, for muscle contractions and for many cellular processes--including signal
transduction. Therefore, plasma calcium concentrations must be maintained within a
narrow normal range of 9–10 mg/dL. PTH functions by increasing blood calcium
concentrations when calcium ion levels fall below normal. Calcium ions bind to specific
receptors on the chief cell and inhibit release of PTH; when the plasma calcium level
falls, PTH secretion is stimulated. PTH stimulates reabsorption of calcium from filtrate
during urine formation, increased absorption in the intestines from digested products
and increased activity of osteoclasts to release calcium (and phosphate) from bone
matrix into the plasma. There is some evidence that it may also indirectly inhibit
osteoblast activity.
A parathyroid adenoma is a benign tumor of the parathyroid gland that can cause an
overproduction of PTH leading to hyperparathyroidism. Hyperparathyroidism results in
hypercalcemia, which can lead to kidney stones. Additionally, increased rate of bone
resorption due to higher osteoclast activity due to this condition may also lead to
osteoporosis. Usually only one of the four parathyroid glands is affected and can be
surgically removed without any adverse effects. However, removal of all the parathyroid
glands will cause an imbalance in blood calcium levels resulting in death.
The adrenal glands help regulate the body’s response to stress, controlling
blood pressure, and maintaining the body’s water, sodium, and potassium levels. The
adrenal glands are associated with the pair of kidneys which are retroperitoneal and
lateral to the spinal column ; one gland is located on the superior surface of each
kidney (hence they are also known as suprarenal glands). The adrenal glands consist of
an outer adrenal cortex (cortex means outer layer) and an inner adrenal medulla (medulla
means middle). Functionally and anatomically the adrenal gland is really two glands
packaged together. The cells of the cortex are endocrine and those of the medulla are
neurosecretory. The cells look very different and embryologically they come from
different tissues. These regions secrete different hormones: the adrenal cortex produces
steroid hormones, and the adrenal medulla produces catecholamine hormones.

The adrenal cortex is the outer layer of the adrenal gland and is made up of
layers of epithelial cells and associated capillary networks. These layers form three
distinct regions that secrete different steroid hormones: the outer zona glomerulosa
produces mineralocorticoids (influence salt and water balance), the middle zona
fasciculata produces glucocorticoids (impact metabolism and inflammation), and the inner
zona reticularis produces androgens (regulate catabolism and sexual
characteristics).
The main mineralocorticoid is aldosterone, which regulates the concentration of ions in
urine, sweat, and saliva. Aldosterone release from the adrenal cortex can be triggered
by a number of things including decrease in blood concentrations of sodium ions, blood
volume, or blood pressure, or an increase in blood potassium levels.
The three main glucocorticoids are cortisol, corticosterone, and cortisone. The
glucocorticoids stimulate the synthesis of glucose from non-glycogen sources, and they
promote the release of fatty acids from adipose tissue. These hormones increase blood
glucose levels to maintain levels within a normal range between meals. They are secreted
in response to ACTH, and levels are regulated by negative feedback.
Androgens are a class of hormones and that affect sexual characteristics. Testosterone
is associated with male sexual characteristics, while progesterone and estrogen are
associated with female reproductive function; androstenedione is an intermediate molecule in
the synthetic pathways of these and many of the steroid hormones.
Example
In situations where the gonads are not functioning the progesterone made in the
adrenal glands does influence sexual characteristics. One of the reasons why
post-menopausal women develop more male characteristics is that the progesterone their
adrenal makes gets converted to testosterone.

The adrenal medulla is the inner layer of the adrenal glands and contains
chromaffin cells, which are large, irregularly shaped cells that are
closely associated with blood vessels. These cells are innervated by autonomic
(involuntary) nerve fibers from the central nervous system, which allows for quick
hormone release.
Chromaffin cells of the adrenal medulla produce epinephrine (adrenaline) and
norepinephrine (noradrenaline). Epinephrine is the primary adrenal medulla hormone
accounting for 75–80 percent of its secretions. Epinephrine and norepinephrine increase
heart rate, breathing rate, cardiac muscle contractions, and blood glucose levels. They
also accelerate the breakdown of glucose in skeletal muscles and stored fats in adipose
tissue.
Both are stored in vesicles or granules in the adrenal medulla, very similar to the way
posterior pituitary cells store the neurosecretory hormones from hypothalamus for
release. The release of epinephrine and norepinephrine into the blood is stimulated by
neural impulses from the sympathetic nervous system. These neural impulses originate
from the hypothalamus in response to stress to prepare the body for the fight-or-flight
response.
Blood Supply to the Adrenal Glands
The adrenal glands have a large blood supply. They
receive arterial blood from the renal arteries, the phrenic arteries, and
suprarenal arteries from the aorta. Arterial blood enters the adrenal glands at
the adrenal cortex and drains into venules in the adrenal medulla. The
suprarenal vein of the right adrenal gland drains into the inferior vena cava,
and the suprarenal vein of the left adrenal gland drains into the left renal
vein.
The pineal gland is located between the cerebral hemispheres of the brain.
It is attached to the roof of the third ventricle in the diencephalon. The pineal gland
consists of secretory cells, called pinealocytes, that secrete the hormone
melatonin. Melatonin is derived from serotonin (a neuropeptide) and is
regulated in response to the light and dark of the environment (the diurnal cycle).
Photons, packets of light, are detected by the retinas of the eyes, which initiate a
nerve impulse that is detected by the pineal gland. This mechanism is similar to the
process in the posterior pituitary or adrenal medulla. The pineal gland synthesizes the
highest levels of melatonin during the night, when light levels are the lowest, and the
increased blood concentrations of melatonin makes us sleepy. Blood concentrations of
melatonin are lowest during the day, when light exposure inhibits the synthesis of the
hormone.
The target cells of melatonin are located in the suprachiasmatic nucleus
(SCN) of the brain. The SCN functions as a biological clock that regulates
physiological processes such as the sleep-wake cycle, appetite, and body temperature.
Melatonin is thought to inhibit the release of gonadotropins from the anterior
pituitary, which affect the onset of puberty.
Higher melatonin levels at night make us sleepy, and a disruption in melatonin synthesis
can disrupt the sleep cycle. Travel across several time zones can result in disruptions
to the sleep cycle, or jet lag. This is due to the change in the dark-light cycle that
the body is accustomed to, and it can take several days for melatonin synthesis to adapt
to the change. Melatonin supplements are available to treat jet lag as well as other
sleep disorders; however, their efficacy has not been conclusively established.
The pancreas is an elongated organ that plays a central role in energy
metabolism, storage, and utilization of glucose (carbohydrate). It is located slightly
dorsal to the stomach and between the stomach and the small intestine. The tapered
distal end lies in contact with the spleen, while the ducts from broader proximal end
enter the duodenum at gastro-duodenal junction. The pancreas contains both exocrine
cells that excrete digestive enzymes and endocrine cells that release hormones.
Approximately 99 percent of pancreatic cells are exocrine cells that are arranged around
ducts in clusters called acini (singular is acinus).
The endocrine cells of the pancreas form clusters called pancreatic islets or the
islets of Langerhans (islets are small islands) with associated blood
capillaries. The pancreatic islets contain two major cell types: alpha
cells, which constitute 20 percent of the total mass of the islets and
produce the hormone glucagon, and beta cells, which constitute 75 percent
of the total mass of the islets and produce the hormone insulin. These hormones regulate
blood glucose levels. Alpha cells release glucagon as blood glucose levels decline below
the set point. When blood glucose levels rise, alpha cells stop secreting glucagon, and
beta cells then release insulin. When blood glucose levels drop below the set point
(which does not happen under normal conditions), beta cells stop secreting insulin.
Pancreatic cells also contain two minor populations of cells: delta cells, which
constitute 4 percent of the total mass of the islets and secrete somatostatin, and F (or
PP) cells, which constitute 1 percent of the total mass of the islets and secrete
pancreatic polypeptide. They have specific paracrine regulator effects in the pancreas.
Pancreatic polypeptide is secreted after a high protein meal or fasting and inhibits
pancreatic exocrine secretion and stimulates gastric juice secretion (opposite effect of
cholecystokinin, CCK, of the small intestine). It also stimulates both alpha and beta
cells.
The gonads, the male testes (sing. Testis) and
female ovaries, function in production of gametes (sperm and ovum) and also produce
steroid hormones. The testes produce androgens, testosterone being the most prominent.
Testosterone stimulates the development of male secondary sex characteristics and the
production of sperm cells. The testes also produce the hormone inhibin, which inhibits
the release of the tropic hormone from the anterior pituitary follicle-stimulating
hormone (FSH) needed for the development of sperm (spermatogenesis).
The ovaries produce the hormones estrogen and progesterone, which stimulate the
development of female secondary sex characteristics, regulate the menstrual cycle, and
prepare the body for childbirth. The ovaries also produce the hormone inhibin, which
inhibits the release of FSH.
The placenta, which supplies the necessary nutrients to the fetus, is also an endocrine
organ. It synthesizes and secretes a number of hormones that are crucial for the
maintenance of pregnancy. The human chorionic gonadotropin hormone (hCG),
that is the basis of urine pregnancy tests is another of the placental hormones. As the
fetus develops, the placenta takes over the production of estrogen and progesterone to
maintain pregnancy. The high level of estrogen facilitates the growth of the uterus and
the mammary glands during gestation. Progesterone is important in suppressing maternal
immune response towards the fetus and inhibiting uterine smooth muscle contraction. The
placenta also produces relaxin, that affects collagen metabolism and
softens the pubic symphysis to facilitate birthing. It also produces
lactogen, which is structurally similar to prolactin and growth hormone
(pituitary hormones), but its role, if any, in human lactation is still being
investigated.


| Endocrine Gland |
Associated Hormones |
Main Effect |
| Pituitary (Anterior) |
Growth hormone |
Promotes growth of body tissues |
|
Prolactin |
Promotes milk production |
|
Thyroid-stimulating hormone (TSH) |
Stimulates thyroid hormone release |
|
Adrenocorticotropic hormone (ACTH) |
Stimulates hormone release by adrenal cortex |
|
Follicle-stimulating hormone (FSH) |
Stimulates gamete production |
|
Luteinizing hormone (LH) |
Stimulates androgen/estrogen production by gonads |
|
Pituitary (Posterior) |
Antidiuretic hormone (ADH; also called vasopressin) |
Stimulates water reabsorption by kidneys |
|
Oxytocin |
Stimulates uterine contractions during childbirth |
| Thyroid |
Thyroxine, triiodothyronine |
Stimulate metabolism |
|
Calcitonin |
Reduces blood Ca2+ levels, primarily in children |
| Parathyroid |
Parathyroid hormone (PTH) |
Increases blood Ca2+ levels |
| Adrenal (Cortex) |
Aldosterone |
Increases blood Na+ levels, and related water conservation by kidneys, Decreases
blood K+ levels |
|
Cortisol, corticosterone, cortisone |
Increase blood glucose levels |
| Adrenal (Medulla) |
Epinephrine, norepinephrine |
Stimulate fight-or-flight response |
| Pineal |
Melatonin |
Regulates sleep cycles |
| Pituitary |
Glucagon |
Increases blood glucose levels |
|
Insulin |
Decreases blood glucose levels |
| Testes |
Testosterone |
Stimulates development of male secondary sex characteristics and sperm
production |
|
Inhibin |
Inhibits secretion of FSH |
| Ovaries |
Estrogen and progesterone |
Stimulates development of female secondary sex characteristics, egg production and preparation of the
body for childbirth |
|
Inhibin |
Inhibits secretion of FSH |
There are several organs whose primary functions are non-endocrine but that also possess
endocrine functions. These include the heart, gastrointestinal tract, kidneys, adipose
tissue, skin, and thymus.
Heart and Cardiovascular System
The heart possesses specialized cardiac muscle cells, which are endocrine cells
in the walls of the atria. These cells respond to increased blood volume by
releasing the hormone atrial natriuretic peptide (ANP). Natrium is
the name for sodium in many languages and is the reason that Na is the chemical
symbol for sodium. High blood volume causes the cells to be stretched, opening
stretch-activated membrane channels, resulting in hormone release. ANP acts on
the kidneys to reduce the reabsorption of Na+, causing Na+
and water to be excreted in the urine. ANP also reduces the amounts of renin
released by the kidneys and aldosterone released by the adrenal cortex, further
preventing the retention of water. In this way, ANP reduces the concentration of
Na+ in the blood and causes a reduction in blood volume and blood
pressure. Another natriuretic hormone, BNP (misnamed brain natriuretic because
it was first isolated from pig brains) from the heart ventricle, enhances the
effect of ANP.
Endothelial cells lining the cardiovascular system also have an endocrine
function. They produce paracrine endothelin, a vasodilator and
stimulator of ANP secretion, and nitric oxide (NO), a vasodilator
and inhibitor of ANP secretion.
Digestive System
The digestive system produces several hormones that aid in digestive and
metabolic homeostasis. Endocrine cells are located in the mucosa of the GI tract
throughout the stomach and small intestine. Hormone secretion is controlled by
receptors monitoring the chemical content of the digestive lumen. Some of the
hormones produced include gastrin (from stomach),
secretin, and cholecystokinin CCK (from small
intestine) that act on the GI tract and accessory organs such as the pancreas,
gallbladder, and liver. They trigger the release of digestive juices that help
to break down and digest food in the GI tract. The GI tract also produces the
hormones glucose-dependent insulinotropic peptide (GIP) (from
stomach) and glucagon-like peptide 1 (GLP-1) (from small
intestine). These hormones are secreted in response to glucose in the intestinal
lumen. GIP and GLP-1 target cells are beta cells in the pancreas, which are
stimulated to release insulin and alpha cells that are inhibited from releasing
glucagon. The stomach also produces ghrelin that mediates hunger,
stimulating appetite and growth hormone release.
The liver, as an accessory organ of the digestive system, also has an endocrine
function in production of insulin-like growth factor (IGFs) and
thrombopoietin (THPO). IGFs work with growth hormone to
regulate cell metabolism while THPO triggers the formation of platelets in the
blood. The liver also produces prohormones (angiotensinogen and calcidiol,
vitamin D) and plasma proteins that transport many of the hormones.
Kidneys and Urinary System
The adrenal glands associated with the kidneys are major endocrine glands, and
the kidneys themselves also possess endocrine functions. Renin ('renal' generally describes aspects of the kidney) is
released in response to decreased blood volume or pressure and is part of the
renin-angiotensin system that is responsible for the formation of angiotensin II
and ultimately leads to the release of aldosterone. Both angiotensin II and
aldosterone then causes the retention of Na+ and water, raising blood
volume. The kidneys also release the steroid hormone calcitriol,
which is the biologically active form of vitamin D that aids in the absorption
of Ca2+. Erythropoietin (EPO) is a protein hormone that
triggers the formation of red blood cells in the bone marrow. EPO is released in
response to low oxygen levels. Because red blood cells are oxygen carriers,
increased production results in greater oxygen delivery throughout the body. The
banned substance EPO had been used by athletes at one point to improve
performance, as greater oxygen delivery to muscle cells allows for greater
endurance. Because red blood cells increase the viscosity of blood, artificially
high levels can cause severe health risks.
Adipose Tissue
Adipose tissue is a connective tissue found throughout the body. It produces the
hormone leptin (Greek “leptos” means “thin”) in response to food intake.
Leptin binds to neuropeptide Y in the CNS neurons,
producing a feeling of satiety after eating, thus affecting appetite and
reducing the urge for further eating. Note that it has the opposite effect of
ghrelin secretion from the stomach, but when leptin levels drop, the brain
detects a state of starvation and the feeling of hunger increases. These two
hormones are the subject of much research related to obesity.
Skin
Skin produces cholecalciferol, which is an inactive hormone form of
vitamin D3. It is formed when cholesterol molecules in the skin, in the form of
7-dehydrocholesterol, are exposed to ultraviolet radiation. Cholecalciferol then
enters the bloodstream and is modified in the liver to form calcifediol.
Calcifediol is then modified in the kidneys to form calcitriol, which is the
active form of vitamin D3. Vitamin D plays an important role in bone
formation.
Bone
Bones not only respond to hormones to maintain blood calcium homeostasis, but
recent research shows they also have an endocrine function. Osteocytes have been
found to produce two hormones (fibroblast growth factor 23 and
osteocalcin) that act on kidney, pancreas and other body
tissues influencing Vitamin D and glucose homeostasis.
Thymus
The thymus is an organ that is found behind the sternum and is most
prominent in infants, becoming smaller in size through adulthood, replaced by
adipose tissue that continues to produce angiogenic factors. The thymus is part
of the immune system, with a role in maturation and immunocompetence of
T-lymphocytes. The thymus also produces a group of hormones called
thymosins, because they were first discovered from the thymus,
but now are understood to be produced by many different tissues in the body.
They appear to have an anti-inflammatory effect and stimulate tissue repair.
Thymosin is also involved in neuroplasticity, and they may have clinical
implications in the treatment of cardiovascular, infectious and autoimmune
diseases as well as cancer.
| Organ |
Associated Hormones |
Main Effect |
| Heart |
Atrial Natriuretic Peptide (ANP) |
Reduces blood volume, pressure, and Na+ concentration |
| Gastrointestinal Tract |
Gastrin, Secretin, and Cholecystokinin |
Aid in the digestion of food |
| Kidneys |
Renin |
Stimulates production of angiotensin II |
|
Calcitriol |
Aids in the absorption of Ca2+
|
|
Erythropoietin |
Triggers the formation of red blood cells in the bone marrow |
| Adipose Tissue |
Leptin |
Promotes satiety signals in the brain |
| Skin |
Cholecalciferol |
Modified to form vitamin D |
| Thymus |
Thymosins |
Aid in the development of the immune system |
Hormones have a wide range of effects and modulate many different body processes. The key
processes that will be examined in this section are hormonal regulation of the excretory
system, the reproductive system, metabolism, blood calcium concentrations, growth, and
the stress response.
We will see how hormones help the body to maintain homeostasis, by integrating different
organ systems.
Maintaining a proper water balance in the body is important to avoid dehydration or
excess water. The water concentration of the body is monitored by
osmoreceptors in the hypothalamus, which detect the concentration of
electrolytes in the blood. The concentration of electrolytes in the blood rises when
there is water loss due to excessive perspiration, inadequate water intake, or low blood
volume due to blood loss. An increase in blood electrolyte levels results in a neural
signal being sent from the osmoreceptors to the hypothalamus. The hypothalamus produces
antidiuretic hormone (ADH), which is transported to, and released from,
the posterior pituitary. It is also known as vasopressin. The target cells for ADH are
the distal tubule cells in the kidneys, which are stimulated to absorb more water from
urine, resulting in an increase in the water level of blood and making urine which is
more concentrated.
Chronic underproduction of ADH results in diabetes insipidus. If the
posterior pituitary does not release enough ADH, water cannot be retained by the kidneys
and is eliminated in the urine. This causes increased thirst, but water taken in is lost
again, and water must be continually consumed. If the condition is not severe,
dehydration may not occur, but severe cases can lead to electrolyte imbalances due to
dehydration.
Another hormone responsible for maintaining electrolyte concentrations in extracellular
fluids is aldosterone, a steroid hormone that is produced by the adrenal
cortex. In contrast to ADH, which promotes the reabsorption of water to maintain proper
water balance, aldosterone maintains proper water balance by enhancing Na+
reabsorption from extracellular fluids. Because it affects the concentrations of
minerals, Na+, aldosterone is referred to as a mineralocorticoid. Aldosterone
release is stimulated by a decrease in blood sodium levels, blood volume, or blood
pressure, or an increase in blood potassium levels. Aldosterone targets the renal
tubules of the kidneys, where it causes the reabsorption of Na+ from urine
and the secretion of K+ into the urine. It also prevents the loss of
Na+ from sweat, saliva, and gastric juice. The reabsorption of
Na+ also results in the osmotic reabsorption of water, which alters blood
volume and blood pressure.
Aldosterone production can be stimulated by low blood pressure, which triggers a sequence
of chemical releases. When blood pressure drops, cells in the juxtaglomerular apparatus
of the kidney detect this and release renin. Renin circulates in the blood
and reacts with a protein produced by the liver called angiotensinogen. When
angiotensinogen is cleaved by renin, it produces angiotensin I, which is then converted
into angiotensin II. Angiotensin II impacts water and Na+ reabsorption and
causes the release of aldosterone by the adrenal cortex with a similar effect;
ultimately these increase blood pressure. Angiotensin II also causes an increase in ADH
and increased thirst, which both help to increase fluids and raise blood pressure.
Regulation of the reproductive system is a process that requires the action of hormones
from the pituitary gland, the adrenal cortex, and the gonads. During puberty in both
males and females, the hypothalamus produces gonadotropin-releasing hormone
(GnRH), which stimulates the release of follicle-stimulating hormone
(FSH) and luteinizing hormone (LH) from the anterior pituitary.
These hormones regulate the gonads (testes in males and ovaries in females) and
therefore are called gonadotropins. In both males and females, FSH
stimulates gamete production, and LH stimulates production of hormones by the gonads. An
increase in gonad hormone levels inhibits GnRH production through a negative feedback
loop.
Regulation of the Male Reproductive System
In males, FSH and LH regulates the maturation of sperm cells. FSH stimulates the
support cells in the testes called Sertoli (sustentacular) cells. These cells nourish and
regulate the maturation of the sperm and produce androgen binding protein (ABP).
ABP keeps the level of testosterone high within the testes, relative to the
plasma levels. FSH production is inhibited by the hormone inhibin,
which is also released by the Sertoli cells in the testes. LH stimulates
production of the sex hormones (androgens) by the interstitial or
Leydig cells of the testes and therefore is also called interstitial
cell-stimulating hormone (ICSH).
The most important androgen in males is testosterone. Testosterone
promotes the production and maturation of sperm and determines secondary sex
characteristics. The adrenal cortex of both males and females also produces
small amounts of testosterone, although the role of this additional hormone
production in males is not well understood.
Regulation of the Female Reproductive System
In females, FSH stimulates development of support cells around the egg, which
develop into structures called follicles. LH regulates the development and
release of the egg from the follicle. Follicle cells initially produce the
hormone estrogen that has effects on the hypothalamus and anterior
pituitary, as well as the uterus. LH stimulates the egg to mature within the growing follicle. As
estrogen levels rise, it triggers the anterior pituitary to release a surge of
LH that stimulates ovulation. The follicle cells remaining in the ovary become
the corpus luteum and now produce both estrogen and progesterone as well as
inhibin, all of which inhibit the anterior pituitary.
Estrogen and progesterone are steroid hormones that prepare the body
for pregnancy. Estrogen produces secondary sex characteristics in females, while
both estrogen and progesterone together regulate the uterine menstrual cycle.
Regulation of the Female Reproductive System and Childbirth
In addition to producing FSH and LH, the anterior pituitary also produces the
hormone prolactin (PRL). In females, prolactin stimulates the production of milk
by the mammary glands following childbirth. Prolactin levels are regulated by
the hypothalamic hormones prolactin-releasing hormone (PRH) and
prolactin-inhibiting hormone (PIH), the latter of which is now known to be
dopamine. Usually prolactin production is inhibited, but PRH, estrogen and infant
suckling stimulation remove the inhibition. Males can produce small amounts of
prolactin and its role in other aspects of reproduction and immunity are not
well understood but are being investigated in humans.
The posterior pituitary releases the hormone oxytocin, which stimulates
contractions during childbirth. The uterine smooth muscles are not very
sensitive to oxytocin until late in pregnancy when the number of oxytocin
receptors in the uterus peaks. Stretching of tissues in the uterus and vagina
stimulates oxytocin release in childbirth. Contractions increase in intensity as
blood levels of oxytocin rise until the birth is complete. Oxytocin also
stimulates the contraction of myoepithelial cells around the milk-producing
cells of the mammary glands. As these cells contract, milk is forced from the
secretory alveoli into milk ducts and is ejected from the breasts in a milk
let-down reflex. Oxytocin release is stimulated by the suckling of an infant,
which triggers the synthesis of oxytocin in the hypothalamus and its release
into circulation at the posterior pituitary.
Blood glucose levels vary widely over the course of a day as periods of food consumption
alternate with periods of fasting. Insulin and glucagon are the two hormones that are
primarily responsible for maintaining homeostasis of blood glucose levels. Additional
regulation is mediated by the thyroid hormones.
Regulation of Blood Glucose Levels by Insulin and Glucagon
Cells of the body require nutrients in order to function, and they obtain these
nutrients through feeding. In order to manage nutrient intake, storing excess
and utilizing stores when necessary, the body uses hormones to modulate
energy metabolism. Insulin is produced by the beta cells of the
pancreas, which are stimulated to release insulin as blood glucose levels rise,
for example, after a meal is consumed. Insulin lowers blood glucose levels by
enhancing glucose uptake by most body target cells, which utilize glucose for
ATP production; muscle cells are a good example. It also stimulates the liver to
convert glucose to glycogen, which is then stored by cells for later use.
Increased glucose uptake occurs through an insulin-mediated increase in the
number of glucose transporter proteins in cell membranes, which remove glucose
from circulation by facilitated diffusion. As insulin binds to its target cell,
it triggers the cell to incorporate transport proteins into its membrane. This
allows glucose to enter the cell, where it can be used as an energy source.
However, this does not always occur in all body cells, as some cells in the
kidneys and brain have been shown to regularly access glucose without the use of
insulin. Insulin also stimulates the conversion of glucose to fat in adipocytes
and the synthesis of proteins. The actions of insulin which cause blood glucose
concentrations to fall, called a hypoglycemic effect, inhibit further insulin
release from beta cells through a negative feedback loop.
Decreased insulin production or reduced sensitivity of cells to insulin can lead
to a condition called diabetes mellitus. This prevents glucose from
being absorbed by cells, causing high levels of glucose in the blood, or
hyperglycemia. High blood glucose levels make it difficult for
the kidneys to reabsorb all the filtered glucose, resulting in glucose being
lost in urine. High glucose levels also result in less water being reabsorbed by
the kidneys because the water is retained in the urine to osmotically balance
the high levels of excess glucose. This causes increased urination, which may
result in dehydration. Over time, high blood glucose levels can cause nerve
damage to the eyes and peripheral body tissues, as well as damage to the kidneys
and cardiovascular system. On the other hand, over-secretion of insulin can lead
to low blood glucose levels, or hypoglycemia. This causes
insufficient glucose availability to cells, often leading to muscle weakness,
and can sometimes cause unconsciousness or death if left untreated.
When blood glucose levels decline below normal levels, for example, between meals
or when glucose is utilized rapidly during exercise, the hormone
glucagon is released from the alpha cells of the pancreas.
Glucagon raises blood glucose levels, called a hyperglycemic effect, by
stimulating the breakdown of glycogen to glucose in skeletal muscle cells and
liver cells in a process called glycogenolysis
(glycogen-splitting). Glucose can then be utilized as energy by muscle cells and
released into circulation by the liver cells. Glucagon also stimulates
absorption of amino acids from the blood by the liver, which then converts them
to glucose. This process of glucose synthesis is called
gluconeogenesis (new glucose formation). Glucagon also
stimulates adipose cells to release fatty acids into the blood. Collectively,
these glucagon-mediated actions result in an increase in blood glucose levels to
normal homeostatic levels. Rising blood glucose levels inhibit further glucagon
release by the pancreas. In this way, insulin and glucagon work together to
maintain glucose homeostasis.
Regulation of Blood Glucose Levels by Thyroid Hormones
The basal metabolic rate, which is the amount of calories required by the body at
rest, is determined by two hormones produced by the thyroid gland:
thyroxine, also know as tetraiodothyronine or T4,
and triiodothyronine, also know as T3. Dietary iodine is
needed to synthesize these hormones. These hormones affect nearly every cell in
the body except for the adult brain, uterus, testes, and spleen. They cross the
plasma membrane of target cells and bind to receptors on the mitochondria,
resulting in increased ATP production. In the nucleus, T3 and
T4 activate genes involved in energy production and glucose
oxidation. This results in increased rates of metabolism and body heat
production, which is known as the hormone’s calorigenic effect.
Disorders can arise from both the underproduction and overproduction of thyroid
hormones. Hypothyroidism, under activity of the thyroid hormones,
can cause a low metabolic rate, leading to weight gain, sensitivity to cold, and
reduced mental activity, among other symptoms. In children, hypothyroidism can
cause cretinism, which causes mental retardation and growth defects.
Hyperthyroidism, the over-activity of thyroid hormones, can
lead to an increased metabolic rate, causing weight loss, excess heat
production, sweating, and an increased heart rate. Graves’ disease is one such
hyperthyroid condition. In the absence of iodine, thyroid hormones are not
produced, colloid storage increases, and thyroid enlargement
(goiter) occurs.
Regulation of blood calcium concentration is important for proper muscle contractions and
release of neurotransmitters. Calcium also affects voltage-gated plasma membrane ion
channels, affecting nerve impulses and other cell physiology. If plasma calcium levels
are too high, membrane permeability to sodium decreases and membranes become less
responsive. If plasma calcium levels are too low, membrane permeability to sodium
increases and convulsions or muscle spasms can result.
Blood calcium levels are regulated by parathyroid hormone (PTH), which is
produced by the parathyroid glands. PTH is released in response to low blood
Ca2+ levels. PTH increases Ca2+ levels by targeting the
skeleton, the kidneys, and the intestine. In the skeleton, PTH stimulates osteoclasts,
causing bone to be broken down and releasing Ca2+ from bone into the blood.
PTH also inhibits osteoblasts, reducing Ca2+ deposition in bone. In the
kidneys and intestines, PTH stimulates the reabsorption of Ca2+. While PTH
acts directly on the kidneys to increase Ca2+ reabsorption, its effects on
the intestine are indirect. PTH triggers the formation of calcitriol, an active form of
vitamin D, which acts on the intestines to increase absorption of dietary calcium. PTH
release is inhibited by rising blood calcium levels.
Example
Hyperparathyroidism results from an overproduction of parathyroid hormone. This
results in excessive calcium being removed from bones and being introduced into
blood circulation. This causes structural weakness of the bones, which can lead to
deformation and breakage, and nervous system impairment due to high blood calcium
levels. Hypoparathyroidism, the underproduction of PTH, results in extremely low
levels of blood calcium, which causes impaired muscle function and may result in
tetany—severe sustained muscle contraction.
The hormone calcitonin is produced by the parafollicular or C cells of the
thyroid and has the opposite effect on blood calcium levels as PTH. Calcitonin decreases
blood calcium levels by inhibiting osteoclasts, stimulating osteoblasts, and stimulating
calcium excretion by the kidneys. This results in calcium being added to the bones to
promote structural integrity. Calcitonin appears to play a major plasma calcium
homeostasis role only in children, and in pregnant women to reduce maternal bone loss.
Its role in adults is not well understood and it may be more important in regulating
bone remodeling than blood plasma homeostasis.
Hormonal regulation is required for the growth and replication of most cells in the body.
Growth hormone (GH) produced by the anterior pituitary accelerates the
rate of protein synthesis, particularly in skeletal muscle and bones. Growth hormone has
direct and indirect mechanisms of action. One direct action is the stimulation of fat
breakdown (lipolysis) and release into the blood by adipocytes. This results in a switch
by most tissues from utilizing glucose as an energy source to utilizing fatty acids.
This process is called a glucose-sparing effect. Another direct action
occurs in the liver, where GH stimulates glycogen breakdown, and subsequent release of
glucose into the blood. Blood glucose levels increase as most tissues are metabolizing
fatty acids instead of glucose. The GH-mediated increase in blood glucose levels is
called a diabetogenic effect because it is similar to the high blood
glucose levels seen in diabetes mellitus.
The indirect mechanism of GH action is mediated by somatomedins or insulin-like
growth factors (IGFs), which are a family of growth-promoting proteins
produced by the liver. IGFs stimulate the uptake of amino acids from the blood, allowing
the formation of new proteins, particularly in skeletal muscle cells, cartilage cells,
and other target cells. GH levels are regulated by two hormones produced by the
hypothalamus. GH release is stimulated by growth hormone-releasing hormone
(GHRH) and is inhibited by growth hormone-inhibiting hormone (GHIH), also
called somatostatin. Both of these are produced by the hypothalamus and delivered to the
anterior pituitary by the hypophyseal portal vein.
Over-secretion of growth hormone can lead to gigantism in children, causing
excessive growth. In adults, excessive GH can lead to acromegaly, a
condition in which bones still capable of growth in the face, hands, and feet enlarge.
Under-production of GH in adults does not appear to cause any abnormalities, but in
children it can result in pituitary dwarfism, in which growth is
proportionally reduced.
When a threat or danger is perceived, the body responds by releasing hormones that will
ready it for the fight-or-flight response. The effects of this response are familiar to
anyone who has been in a stressful situation: increased heart rate, dry mouth,
butterflies in your stomach and sweating. This is what we call a short-term stress. When
one is under stress for more than several hours (or chronically), it translates into
long term stress.
The sympathetic nervous system regulates the stress response via the hypothalamus.
Stressful stimuli cause the hypothalamus to signal the adrenal medulla via nerve
impulses, which mediates short-term stress responses, and to the adrenal cortex, via the
hormone adrenocorticotropic hormone (ACTH), which mediates long-term stress
response.
Short-Term Stress Response
Upon stimulation, the adrenal medulla releases the hormones
epinephrine (also known as adrenaline) and
norepinephrine (also known as noradrenaline), collectively
referred to as the catecholamines. The release of these hormones into the blood
provides the body with a burst of energy that is needed to respond to a
stressful situation. Epinephrine and norepinephrine increase blood glucose
levels by stimulating the liver and skeletal muscles to break down glycogen and
by stimulating glucose release by liver cells. These hormones also increase
oxygen availability to cells by increasing the heart rate and dilating the
bronchioles. In addition, they increase the blood supply to essential organs
such as the heart, brain, and skeletal muscles by stimulating specific blood
vessels to relax or by constricting other vessels to divert blood away from
nonessential organs such as the skin, digestive system, and kidneys.
Long-Term Stress Response
In a long-term stress response, the hypothalamus triggers the release of ACTH
from the anterior pituitary. The adrenal cortex is stimulated by ACTH to release
steroid hormones called corticosteroids. The two main
corticosteroids are glucocorticoids, such as cortisol,
and mineralocorticoids, such as aldosterone. The glucocorticoids
primarily affect glucose metabolism by stimulating glucose synthesis. They also
stimulate the redistribution of fat stored in adipose tissue for use in meeting
long-term energy requirements. Glucocorticoids also have anti-inflammatory
properties through inhibition of the immune system. For example, cortisone is
used as an anti-inflammatory medication; however, it cannot be used long term as
it increases susceptibility to disease due to its immune-suppressing effects as
well as changes to blood pressure and constant heart stimulation.
Mineralocorticoids function to regulate ion and water balance of the body. The
hormone aldosterone stimulates the reabsorption of water and sodium ions in the
kidney, which results in increased blood pressure and volume.
Hypersecretion of glucocorticoids, unrelated to a normal stress response, can be
caused by a condition known as Cushing’s disease. This can cause
the accumulation of adipose tissue in the face and neck and excessive glucose in
the blood. Hyposecretion of the corticosteroids, independent of the normal
stress response, is often the result of Addison’s disease, which
may result in low blood sugar levels and low electrolyte levels.
Tumors
Endocrine glands tightly regulate hormone synthesis and release to maintain
homeostasis in the body. Although they are rare, tumors in endocrine glands do
occur, disrupting normal hormone synthesis. These cancers, called neuroendocrine
tumors or NETs, affect cells of the endocrine and nervous system that control
hormone synthesis.
Tumors of endocrine glands can produce symptoms that are similar to those caused
by hypersecretion of hormones in endocrine disorders. For example, tumors of the
adrenal glands that result in the excess production of steroid hormones produce
symptoms of Cushing’s disease, which includes the accumulation of adipose tissue
in the face and neck and excessive glucose in the blood.
Tumors of the pancreas that affect beta cells are called insulinomas, and those
that affect alpha cells are called glucagonomas. Beta cell tumors result in the
production of excess amounts of the hormone insulin, which results in
hypoglycemia. Alpha cell tumors result in the production of excess amounts of
the hormone glucagon, which results in hyperglycemia and symptoms similar to
those seen in diabetes.
Tumors of the pituitary gland fall into two groups: secreting and non-secreting
tumors. Secreting tumors cause the secretion of excess amounts of pituitary
hormones. Symptoms of secreting pituitary tumors are related to the function of
the hormone that is affected. For example, growth hormone-producing tumors can
lead to gigantism and acromegaly.
Medullary thyroid cancer (MTC) is a cancer of the parafollicular cells of the
thyroid gland. The parafollicular cells produce the hormone calcitonin, which
plays a role in calcium regulation and bone formation. Unlike in other cancers,
the increased levels of calcitonin produced by MTC in adults are not harmful.
Increased blood calcitonin levels are used to diagnose MTC.
Many secretory NETs can be tentatively diagnosed by measuring blood hormone
levels to identify hormones that are present in excess. Treatment of these
tumors can include surgery to remove the tumor or affected endocrine gland and
chemotherapy.
Osteoporosis
Osteoporosis is a disease of the skeletal system characterized by a decrease in
bone density and deterioration of bone tissue. The mechanism of disease onset is
an imbalance between bone formation and bone resorption, with bone resorption
occurring at a greater rate than bone formation. Hormone levels play a vital
role in maintaining a balance in bone turnover. Osteoporosis affects primarily
the elderly and is three times more common in women than in men. This may be
related to lower peak bone mass in women and to hormonal changes that occur
during menopause. Low estrogen levels increase the rate of bone turnover, which
alters the balance between bone formation and bone resorption. The decrease in
estrogen that occurs during menopause appears to be the primary cause of
osteoporosis in women over 50 years of age.
The elderly also have decreased levels of the hormone calcitriol, which is the
active form of vitamin D3. Vitamin D helps maintain calcium balance
in the skeleton by stimulating calcium absorption in the intestines and by
maintaining calcium and phosphate levels for bone formation. Vitamin D also
helps to maintain homeostatic levels of parathyroid hormone (PTH). Decreased
vitamin D levels are associated with increased PTH levels, which result in
increased bone turnover and bone loss.
Decreased dietary intake of calcium in the elderly is also associated with
osteoporosis. In addition to the decreased availability of calcium for bone
formation, low blood calcium levels stimulate the parathyroid glands to
synthesize more PTH. PTH stimulates the release of calcium from bones to maintain
blood calcium levels, resulting in increased bone loss.
Osteoporosis can be prevented by maintaining a lifestyle that includes exercise
and proper nutrition. It can be treated using the hormone calcitonin, which is
normally produced by the parafollicular cells of the thyroid gland. Calcitonin
functions to lower blood calcium levels and promotes bone formation. Calcium and
vitamin D supplements have also been shown to reduce the incidence of
osteoporosis.
The digestive system is basically a tube within our body, from mouth to anus. It includes
the stomach and intestines, as well as accessory organs such as the liver, gall bladder
and pancreas. The space within any tubular body structure, such as blood vessels or this
digestive tract, is known as a lumen. During embryological development, embryonic cells
(endoderm) forming the primitive yolk sac, turn outside-in (invaginates) forming the
anal opening to the outside. This endoderm layer becomes the epithelial tissue lining of
the future gastrointestinal (GI) tract and many associated organs. Later in the
development process, the mouth opening breaks through the outer layer of embryonic cells
(ectoderm) from the opposite side, meeting the endoderm. This ectoderm lines the mouth
and forms the salivary glands. As a result, anything inside the space or lumen of the GI
tract can technically be described as still being “external” to the body tissues. So, the
caustic process of digestion is occurring in an “external” tube, without destroying the
“internal” body tissues themselves. Then the products of digestion are absorbed from
this “external environment” into the body cells and tissue fluids.
As early as the 2nd century, early anatomists understood the basic structure and
important function of the digestive system. Many of the terms we still use for anatomy
were developed then and during the medieval period, when they described the importance
of the stomach and intestines for proper nutrition and digestion to maintain health. They
also recognized the association of the liver and gall bladder to produce and store bile,
although they didn’t correctly understand its physiology. It was identified as one of
the four body fluids (blood, phlegm, yellow bile and black bile) that Hippocrates
proposed were the physiological foundation for different human emotions and behaviors;
thus they were called “humors”. The imbalance of these four humors was thought to be the
cause of physical and mental illnesses. This and many of the early physiological
theories have since been discarded. However, their idea that the stomach was an animate
organ with ability to think or feel doesn’t seem totally ridiculous as you learn about
how modern science is discovering the physiological importance of a specific part of the
nervous system that resides entirely in our gut. By the renaissance period,
physiologists were focusing their research on the chemical basis of digestion occurring
inside the GI tract.
Several of the core ideas in A&P are clearly demonstrated as you investigate the
digestive system in this module:
-
Living organisms are causal mechanisms whose functions are to be understood by
the applications of the laws of physics and chemistry.
Both mechanical and chemical digestion are necessary to convert the food we eat
into a form that can be absorbed.
-
The cell is the basic unit of life and cell plasma membranes control transport
and signaling necessary for life.
Specialized cells are able to secrete
substances into the digestive system space (lumen), to protect the body from
this mixture because some of these substances would otherwise digest the body,
and then to absorb specific substances from the mixture into the body.
-
Life requires information flow within and between cells and between the environment and the organism.
Receptors monitor the composition of the mixture as it moves
through the digestive system lumen, stimulating secretion of hormones
and nerve impulses that coordinate the release of substances and control
the movement from one segment to another by circular muscles called
sphincters.
-
Living organisms must obtain matter and energy from the external world. This matter and energy must be transformed and transferred in varied ways to build the organism and to perform work.
Food in the diet must be broken down into its basic units for
absorption and then these are used to fuel ATP production, build and
repair cells and tissues, and participate in other metabolic activities
of the body.
-
Homeostasis (and “stability” in a more
general sense) maintains the internal environment in a more or less
constant state compatible with life.
In addition to nutrients, the absorption of vitamins,
electrolytes and water are critical to maintaining body homeostasis,
with disorders and diseases occurring when malabsorption occurs.
-
Understanding the behavior of the organism requires understanding the relationship between structure and function (at each and every level of organization).
This is best illustrated by the concept of increased surface area for efficient absorption in the digestive system.
-
Living organisms carry out functions at many different levels of organization simultaneously.
Cell level function is supported by all the tissue structures
that make up the different segments and organs of the digestive system.
-
All life depends upon the proper interactions and supporting functions among interrelated organ systems.
In addition to obtaining necessary metabolic substances needed by
all other body systems, the digestive system has several mechanisms to
protect other systems against pathogens that can enter with things we
ingest.
Here is a preview of each of the modules to come:
-
Structures and Functions, will explore how the major organs of the digestive system are able to accomplish the major system level functions:
- movement through the length of the digestive system
- complementary mechanisms for digestion in different segments
- structural components enhancing efficient absorption
Pay attention to not only what structures are similar and
what are unique in the different sections, but how this contributes to
the overall function of the system as a whole.
-
Levels of Organization, will examine this
structure and function in more detail, progressing through the major
levels of organization in the human organism from the chemical and
cellular levels to the tissues, organs and organ systems:
- nutritive and digestive molecules
- cells of the stomach, small and large intestine, liver, and pancreas
- tissue layers and organs that make up the GI tract, from mouth to anus
- accessory organs that contribute secretions to the GI tract
Pay attention to the function of cells for both secretion
and absorption of specific substances in the different parts of the
digestive tract and accessory organs. What these specific cells produce
and how they move materials across cell membranes is critical to
understanding the function of the system as a whole.
-
Homeostasis, will delve into how this system contributes to the body’s natural tendency to maintain a stable internal environment:
- how hormones and nerves control and coordinate the digestive system
- what happens when digestive system malfunctions
Pay attention to how not only is the rate of movement from
one section to another controlled, but what is released into the lumen
is controlled based on monitoring of content of food eaten. Don’t get
the endocrine secretion of hormones into the blood for control and
regulation confused with the exocrine secretion of substances into the
lumen for the digestion and absorption processes – both are happening.
-
Integration of Systems, will investigate which systems are subsets of larger systems, and how they function together in harmony and conflict:
- how digestive system interacts with the other body systems
- how other systems affect the digestive system functions
Pay attention to the relationship between proper movement
and secretion for digestion and the subsequent absorption and transport
of materials for metabolism. This links the digestive tract and
accessory digestive organs to the other systems.
The digestive system moves water, nutrients and electrolytes from the external
environment to the internal environment. Within the digestive system, the
gastrointestinal tract is a continuous hollow tube from the mouth to the anus and is
technically contiguous with the external environment. This internal space is called a
lumen. All digestive system organs play vital roles in breaking down food moving through
the gastrointestinal tract into its chemical building blocks, absorbing these building
blocks into the blood, and eliminating residual indigestible materials.
Different parts of the digestive system will secrete substances into the digestive tract
lumen. These secretions come from both the cells that make up the epithelial lining of
the digestive tract and from exocrine accessory organs that have ducts emptying into the
tube. These substances will assist with the movement of food from one part of the tract
to the next (such as mucus) and with the digestion of the food while it is in the tube
(such as enzymes).
In order to absorb digested substances, that material has to be first moved from the
lumen, into the apical or tube-side of the epithelial cells lining the digestive tract,
then out of the other basal side of those cells, into the body’s interstitial fluid, and
into blood or lymph capillaries for transport through the body. This process can happen
passively or require ATP cellular energy. You may want to review membrane transport in
the cell module. Various chemicals secreted into the lumen can enhance the absorption
of some material, such as vitamins and lipids. Anatomical structures that increase the
area of the absorptive surface exposed to the digested substances in the tube will also
enhance absorption.
learn by doing
Many different digestive dysfunctions have very similar symptoms. Understanding
the biochemical and cellular functions of the digestive system can help in
understanding some of the various causes.Celiac disease is a digestive system
disorder that is also known as gluten intolerance. Gluten is made up of amino
acids and is found in wheat. When the enzyme transglutiminase reacts with gluten
in the digestive system, it can cause an autoimmune response that destroys the
lining of the small intestine. This causes improper absorption and associated
symptoms.
There will be another kind of secretion involved in regulating this whole process,
including how long food stays in one section of the digestive tract for the most
efficient digestion or absorption. These are endocrine secretions from cells that are
part of the digestive organs themselves. These hormones are secreted into the blood, not
the digestive lumen, and transported by blood to target cells of the digestive system
organs. In this way, it helps regulate and coordinate the functions among the different
organs. The autonomic nervous system also plays a role in regulating and coordinating
the digestive system.
learn by doing
Some people with Panic Disorder have been found to have an inherited metabolic
defect that prevents them from producing a digestive system hormone called
cholecystokinin (CCK). CCK controls the release of digestive secretions from the
liver/gall bladder and pancreas into the small intestine. CCK also acts as a
central nervous system neuropeptide, affecting neuron communication in parts of
the brain that regulate anxiety and stress. People with this particular genetic
condition may experience Gastro-Esophageal Reflux Disease (GERD) or Irritable
Bowel Syndrome (IBS), sometimes for years before they ever have a panic attack
experience.
We usually don't think about the digestive system until something goes wrong with it –
when we get indigestion, nausea, or diarrhea. We seldom appreciate that the digestive
system is a complex string of organs that make up the body's engine, turning fuel from
the food we eat into energy that keeps us going. Each organ of the digestive system
performs specific functions but all these organs work together to digest the foods we
eat and absorb the nutrients into our bodies. Feel free to review the overall
Digestive
system in the course Introduction.
learn by doing
Lactose intolerance is a digestive system disorder related to the
biochemical processes. In this genetic disorder, the person doesn’t produce
enough lactase to digest lactose in their diet, so lactose isn’t absorbed in the
small intestine. When it moves on to the large intestine, lactose upsets the
water homeostasis and bacteria can metabolize it, creating the various
symptoms.
The digestive system is generally divided into two main categories: organs of the
alimentary canal (aliment = “nourish”) and accessory digestive
organs. The alimentary canal, also called the gastrointestinal (GI)
tract or gut, is a continuous muscular tube that runs from the
mouth to the anus. The internal space of this tube is called the lumen. The
GI tract is involved with the digestion of food –its breakdown into smaller fragments –
and the absorption of digested food fragments from the lument through the alimentary
canal wall and into the bloodstream. The accessory digestive organs contribute to
secretions to the GI tract, but the food doesn't pass through these organs.
 Digestive System organs. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Digestive System organs. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Different digestive system organs are responsible for different digestive processes and
functions. These functions include: extracting nutrients from food and removing waste.
The processes by which these occur are called ingestion,
motility of food through the GI tract lumen, mehcanical
and chemical digestion of the food, absorption and breakdown
of products, defecation to remove residues.
| Organ |
Major Functions |
Other Functions |
| Mouth |
Ingests food; Mechanical chewing of food; Salivary amylase begins
chemical breakdown of starch; Swallows food and propels it into pharynx |
Salivary mucus helps dissolve food; Release of flavors stimulates
tastebuds allowing us to appreciate its taste; Saliva moistens
food, and tongue helps create a bolus that can be swallowed; Saliva
cleans and lubricates the teeth and oral cavity |
| Pharynx |
Propels bolus from oral cavity to esophagus |
Mucus lubricates food passageways |
| Esophagus |
Peristaltic waves propel food bolus to stomach |
Mucus lubricates food passageways |
| Stomach |
Peristaltic waves combine food with gastric juice and move it into
the duodenum; Pepsin begins protein digestion; Absorbs
some fat-soluble substances (e.g., alcohol, aspirin) |
Hydrochloric acid neutralizes ingested pathogens and stimulates
protein-digesting enzymes; Mucus lubricates and protects the stomach;
Intrinsic factor allows vitamin B12 to be absorbed in
intestines |
| Small intestine |
Mixes contents with digestive juices for digestion and absorption;
Brush-border enzymes digest food; Absorbs breakdown products of
carbohydrates, protein, fat, and nucleic acid digestion, along with vitamins,
water, and electrolytes |
Alkaline mucus helps neutralize acidic chyme from the
stomach |
| Large intestine |
Enteric bacteria digest some food residue and vitamins; Absorbs
most residual water, electrolytes, and vitamins produced by enteric bacteria;
Propels feces toward rectum; Defecation reflex eliminates
feces |
Residues are concentrated and temporarily stored prior to defecation; Mucus
smoothes passage of feces through colon |
| Accessory organs |
Liver: produces bile; Gall bladder: stores and
concentrates bile; Pancreas: produces enzymes that digests food |
Gall bladder releases bile, which emulsifies fat and stimulates the digestion of
fat and the absorption of fatty acids, monoglycerides, cholesterol,
phospholipids, and fat-soluble vitamins; Bicarbonate-rich pancreatic
juice helps neutralize acidic chyme (from the stomach) and provide
optimal environment for enzymatic activity |
Both voluntary skeletal muscles and involuntary smooth muscles are involved in propelling
food material through the digestive system. Skeletal muscles of the tongue are involved
in the voluntary first phase of swallowing, but then it becomes an involuntary reflex
with skeletal muscles of the pharynx. You will learn more about this swallowing process
called deglution in the next module.
The smooth muscle of the GI tract is arranged in two layers: longitudinal along the
length of the tube and circular around the diameter of the tube. Coordinated contraction
and relaxation of these two layers creates a wave-like propulsion of the food called
peristalsis. Muscles in the esophagus mechanically transport the food via peristalsis from the mouth
to the stomach. An extra smooth muscle layer in the stomach adds a churning movement
within the stomach. Peristalsis continues to propel food through the small and large
intestines.
Controlling the rate of food moving from one organ of the digestive tract to the next are
specialized circularly arranged skeletal and smooth muscles called sphincters.
Sphincters are typically contracted and relax only to allow food to move from one organ
to the next. The majority of sphincters along the length of the GI tract are involuntary
smooth muscle structures. The esophageal sphincter helps separate the
esophagus from the stomach to maintain linear movement and protect the esophagus from
acidic chemicals of the stomach. The pyloric sphincter separates the stomach from the small intestine; again
there is a physical separation for compartmentalization and to maintain directionalized
movement.
Material then snakes its way through the small intestine and the iloeocecal
sphincter controls movement into the large intestine or colon. The anal
sphincter muscle is skeletal muscle controlled consciously to relax during
defecation. There is also a hepatopancreatic sphincter that controls the release of
accessory secretions from the liver and pancreas into the beginning of the small
intestine.
Digestion is accomplished both mechanically and chemically. Mechanical digestion
physically breaks food into smaller pieces, increasing surface area for more efficient
chemical digestion. Chemical digestion breaks large food macromolecules down into their
chemical building blocks, which can then be absorbed through the intestinal wall and
into the general circulation.
Food is ingested in the mouth where it begins mechanical digestion from the grinding of
the teeth and chemical digestion from enzymes in the saliva. Chemical digestion starts
in the saliva and follows into the stomach. In the stomach material is broken down into
smaller components by chemically strong acids, enzymes and mechanical churning. However,
the small intestine is the site of most chemical digestion, and almost all absorption.
Enzymes, bile, bicarbonate and other materials are mixed in from the pancreas and liver
to promote chemical digestion and allow enhanced adsorption. Enzymes are
responsible for the majority of this chemical digestion. The breakdown of fat also requires
emulsification by bile, secreted by the liver and stored in the gall bladder.
The mechanical and chemical digestive processes that begin in the mouth and continue
through the small intestine have one endpoint: to convert food into substances that can
be absorbed from the lumen by epithelial cells in the lining of the GI tract and then
enter blood or lymphatic vessels. Absorbable substances are the monosaccharides,
glucose, galactose, and fructose from carbohydrates; single amino acids, dipeptides, and
tripeptides from proteins; and monoglycerides, glycerol, and fatty acids from lipids
such as triglycerides.
Most nutrients are absorbed into digestive epithelial cells by active transport
mechanisms. Nutrients not absorbed through active transport include
lipids, lipid-soluble vitamins, and most water-soluble vitamins. With the help of bile
salts, the breakdown products of lipids are transported into intestinal cells in
structures called micelles. These absorbed fats then aggregate into chylomicrons for
transport.
These substances absorbed into the epithelial cells of the digestive tract are then
absorbed into either the circulatory or lymphatic capillaries for transport where needed
by body cells for metabolism. Absorption from the GI lumen is enhanced by increasing the
surface area. Most absorption occurs in the small intestine, the longest organ of the GI
tract. Several anatomical structures at the organ, tissue, and cell level also improve
nutrient, water and electrolyte absorption.
Plicae circulares
On the organ scale, the inside lining of the small intestine lumen is not smooth.
Plicae circulares, are permanent ridges or folds forming
successive rings along the length of the small intestine. These folds increase
the surface area of the lining exposed to contents in the lumen for a given
length of small intestine, which allows increased absorption.
Villi
At the microscopic tissue level, there are finger-like extensions of the
epithelial and connective tissue called villi. The villi increase
the surface area of the epithelial tissue exposed to contents moving through the
lumen. This allows more superficial epithelial cells to absorb within a square
millimeter of the lining than if this same millimeter surface were flat. Within
each villi, deep to the epithelial basement membrane, the connective tissue
supports the villi structure as it extends into the lumen. Capillary networks
that pick up and transport absorbed substances through the body extend from the
deeper connective tissue into each villus (singular of villi). These include
blood capillaries and a central specialized lymph capillary, called a
lacteal, for lipid absorption.
Microvilli
At the cellular level, the apical surface of each epithelial cell has cell
membrane extensions into the lumen called microvilli. These cells are
collectively known as brush border cells because their combined microvilli along
the surface of each villus looks like the surface of a brush under the
microscope. Because of the microvilli, each epithelial cell has a longer cell
membrane exposed to the lumen contents than it would if its apical surface were
smooth, improving cellular absorption. Embedded in the cell’s folded plasma
membrane are different cell membrane proteins: transporters and enzymes. The
transporters absorb by active transport, but the brush border enzymes are
catalysts that complete the digestion of only partially digested proteins and
carbohydrates at the cell surface, so they can pass through the adjacent
transporters.
Surface Area
Explanation of increased surface of the small instestine due to the characteristics of its structures.
In order to get the various materials needed for energy production and anabolic
metabolism in all body cells, our body has to exchange nutrients, water and electrolytes
from the environment. However, the substance taken into the space of the GI tract is not
always in the form that can be absorbed, so it must first be broken down. Various
secreted chemicals are critical to accomplishing this digestion. These ingested
macromolecules are digested in different regions of the GI tract, requiring that the
mixture of material be moved through the digestive system in a controlled way. Once it
has been digested to a basic chemical structure, the material can be absorbed into the
blood or lymph. At that point, the other body systems integrate to distribute these
absorbed nutrients, fluids and electrolytes through the body as needed. Any material
remaining in the GI tract as waste will then be removed from the body by defecation.
To understand these system-level functions, you will be further exploring structures and
processes occurring at all levels of organization. Some examples of major digestive
structures assigned to their structural level of organization of the digestive system
include:
-
Chemical level - sodium, potassium, chloride, bicarbonate, and phosphate ions and hydrochloric acid
-
Macromolecular level - enzymes of digestion, bile, and the nutritive molecules such as carbohydrates, protein, lipids and nucleic acids
-
Cellular level – mucosa cells, secretory cells, and immune cells
-
Tissue level – epithelial, connective, and smooth muscle tissues
-
Organ level - gastrointestinal tract and accessory organs
-
Organ System level - integration of organs for nutrient and waste movement, digestion and absorption
The digestive system breaks down ingested material into absorbable components (nutrients)
that the rest of the body can use to maintain adequate function. We require a diverse
set of nutrients to support energy production, carry out metabolism and maintain
structure.
Chemical digestion generally, breaks down large food molecules into their respective
chemical building blocks, called monomers, that can be absorbed. The enzymes responsible
for chemical digestion are released by both intrinsic glands found in the
gastrointestinal tract and the accessory glands which secrete molecules into the
gastrointestinal tract.
Hydrolysis refers to the breakdown of a chemical where water breaks a covalent bond.
Hydrolysis can happen spontaneously, but the breakdown of food into macromolecules and
monomers occurs by enzymatic hydrolysis - where the hydrolysis reaction catalyzed by an
enzyme. Hydro- is part of the name because a water molecule is added to each molecular
bond that is broken, or -lysed. More specifically, hydrolysis is a method of degradation
which splits a molecule of water to help break chemical bonds in a larger molecule.
Usually an H+ is attached to one of the components and an OH- group to the other.
Hydrolysis reactions are important for many other physiologic processes in the body in
addition to the breakdown of food molecules.
 Hydrolysis of a Sucrose molecule into Glucose and Fructose.
Hydrolysis of a Sucrose molecule into Glucose and Fructose.
For a review of nutritive molecules, including chemical structure and bonds, revisit the
Chemical Bonding and
Molecules page and the rest of the Levels of Organization unit.
The nutritive organic compounds in our food includes carbohydrates, proteins and lipids.
These molecules are digested, then absorbed, and reassembled into macromolecules or used
as fuel for metabolism in the body. The process of breaking down these macromolecules
involves splitting into smaller molecules using water molecule, thus the name hydrolysis
(“hydro” = water; “lysis” = splitting). The reverse reaction, called dehydration
synthesis, can build macromolecules from the absorbed building blocks. Enzymes can speed
up the rate of these reactions.
Carbohydrates
Our daily food intake usually includes from 200 to 600 grams of carbohydrates.
Carbohydrates are primarily used for quick energy, but some are also used to
create important signaling and structural molecules. The monomers of
carbohydrates, monosaccharides (simple sugars), are absorbed easily
and can therefore be a source of quick energy for the body when we consume them
in this form. Glucose, galactose, and
frutose are the three common monosaccharides in our diet.
 Hydrolysis: carbohydrate digestion
Hydrolysis: carbohydrate digestion
Disaccharides are two monosaccharides bound together and include
sucrose (table sugar), lactose, and
maltose. Polysaccharides are long chains of
monomers and include polymerized glucose in different forms including
glycogen, which is the stored form of glucose in our bodies,
and starch, a polysaccharide of glucose molecules that comes from
plant sources.
In many places around the world, starch accounts for the largest portion of
digestible carbohydrates in the diet, with the addition of some glycogen,
disaccharides and monosaccharides. There are other polysaccharides in our diet,
like cellulose, but our bodies do not produce enzymes that can break them down,
so they are indigestible. While indigestible polysaccharides do not give us any
nutrients, they do provide bulk (fiber) that helps propel food through the
digestive system.
Example
Lactose Intolerance
Individuals with lactose intolerance are unable to metabolize lactose, a sugar found
in milk. The problem is usually caused by a lack of the enzyme lactase, which is
required to break down lactose in the lining of the small intestine. Without
breakdown in the small intestine, lactose flows into the large intestine where
bacteria metabolize lactose in a process called fermentation. In fermentation,
gasses are produced that cause abdominal bloating. Sugars and fermentation products
also cause large amounts of water to enter the large intestine, leading to loose
stool. Most infants are born the enzyme lactase, and as adults lose the ability to
produce this enzyme.
Proteins
We get protein when we eat meat, seafood, eggs, beans, nuts and soy products.
USDA recommends 5 to 6 ounces of protein in diet per day, although children need
less. This dietary protein is usually in the form of polypeptides and must be
digested into its amino
acid building blocks for absorption. There are numerous
enzymes that break large proteins into smaller peptides and then into amino
acids. Amino acids are then absorbed from the digestive system into the
circulatory system where they are delivered throughout the body. Once amino
acids have entered cells throughout the body, they are bonded together to make
proteins needed for cell function. As a last resort, proteins and amino acids
can also be used as energy; they are metabolically converted to glucose before
they are used as energy sources.
 Hydrolysis: protein digestion
Hydrolysis: protein digestion
Lipids and Fats
Dietary lipids include fats and oils. While not considered a USDA food group,
oils contain some essential nutrients and are recommended as part of a healthy
diet, although only in small amounts. Solid fats have more saturated and
trans-fatty acids and are considered empty calories when included in a diet
because they add calories but not needed nutrients. Most dietary lipids are in
the form of triglycerides, with one glycerol molecule and three fatty acids
bound together. Lipids are processed by enzymes secreted from the pancreas
(with some enzymes from the stomach and saliva) and are then solubilized for
absorption by salts secreted in bile (from the liver). These steps prepare them
for absorption in the small intestine. Like proteins, ingested lipids are broken
down into smaller parts for absorption and then are either metabolized to make
energy or are use to make cellular structures including cell membranes. Lipid
absorption is also required for absorption of some fat-soluble vitamins.
 Hydrolysis: lipid digestion
Hydrolysis: lipid digestion
Nucleic Acids
When we eat any plant or animal foods, we are able to digest the DNA or RNA
nucleic acid that was in their cells. The fundamental unit of nucleic acids is
the nucleotide, with a phosphate group, a pentose sugar, and one of 5 nucleic
bases. The pancreas produces and releases into the small intestine two enzymes
to break down either DNA (deoxyribonuclease) or RNA (ribonuclease) into
individual nucleotides. Brush border enzymes of the cells lining the small
intestine further digest the nucleotide into its molecular components for
absorption.
Some salivary glands are always secreting saliva. Intrinsic salivary glands of the mouth
secrete small amounts of saliva, usually just enough to moisten the mucous membranes and
clean the mouth and teeth. The accessory salivary glands are exocrine organs under
autonomic nervous system control with ducts leading into the mouth. Secretion increases
when there is food in the mouth, as well as through a reflex when food is smelled, seen
or thought about (you have probably heard about Pavlov’s experiment with the salivating
dog and bell). Saliva is needed to moisten and lubricate the food and begin its chemical
breakdown.
Saliva is mainly water, which dissolves chemicals in the food. Only dissolved
chemicals can activate the different kinds of taste receptor cells on the tongue, palate
and other parts of the mouth and pharynx. Mixed in with the water, saliva also contains
mucus, various electrolytes typically found in blood plasma, as well as some digestive
molecules, metabolic waste products and immune molecules. Each of these contributes to
the various functions of saliva.
Bicarbonate and phosphate ions help maintain the pH of
saliva as neutral or slightly basic (average 7.4 pH). This not only helps protect the
teeth from acidic substances eaten and plaque bacteria that grow best in an acidic
environment, but also maintains the optimal pH for the enzyme amylase. Salivary amylase
starts breaking complex carbohydrates eaten, like starch, into sugars. This enzymatic
activity will stop once the mixture of food and saliva, called a bolus, is swallowed and
reaches the acidic environment of the stomach. Salivary lipase is another enzyme in
saliva that breaks down dietary lipids, but it is activated when it reaches the acidic
gastric juice with its optimal pH being much more acidic than the salivary pH.
In addition to the water of saliva, mucus in the saliva is composed of glycoproteins and
helps moisten the food, so it can be manipulated into a mass for swallowing. It also
helps lubricate the movement of food through the pharynx and esophagus by peristalsis.
Lysozyme antimicrobial enzymes and antibodies (Immunoglobulin A) in the saliva help
combat pathogens that might enter the body with food or drink. Like the sweat glands,
salivary glands play a role in some metabolic waste excretion, so urea and ammonia are
also found in saliva.
Enzymes and Acids of the Stomach
Gastric glands produce hydrochloric acid (HCl), enzymes, and
intrinsic factor. HCl is responsible for the high acidity
(pH 1.5 to 3.5) of the
stomach contents. The acidity directly kills a lot of the bacteria we ingest
with food and helps proteins and substances found in plants. In the presence of gastric
juice’s low pH, the inactive enzyme, pepsinogen, is modified to
become the active protein enzyme, pepsin. Protein enzymes of the
digestive system are produced and secreted in an inactive form, to be activated
only in the lumen, thus preventing digestion of the cells themselves. The
glycoprotein intrinsic factor is necessary for the absorption of
vitamin B12 later in the small intestine.
It is amazing that cells of the body can produce acids that normally would
destroy the cell itself. Strong acids are also produced in cells in the immune
system (to break down foreign organisms) and the skeletal system (to break down
mineralized bone). High levels of acidity in the wrong places can be very
destructive to living cells. Our body has several mechanisms to control pH.
In the stomach cell, the enzyme carbonic anhydrase converts one molecule of
carbon dioxide and one molecule of water indirectly into a bicarbonate ion
(HCO3-) and a hydrogen ion (H+). In the
stomach ion exchange is used to move H+ ions out the cells and into
the lumen of the stomach.
 Production of Hydrochloric Acid (HCl). This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Production of Hydrochloric Acid (HCl). This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The bicarbonate ion (HCO3-) is then exchanged for a chloride ion
(Cl-) on the basal side (away from the lumen) of the cell. Potassium
(K+) and chloride (Cl-) ions diffuse into the secretory region
of the cell called the canaliculi. Then the potassium is exchanged in this
region for hydrogen ions via a H+/K+ ATPase. The hydrogen ions, which are millions of
times more concentrated in this region of the cell than any other, are then pumped from
the canaliculi into the lumen of the stomach. The following video illustrates this
process.
Production of Hydrochloric Acid in the Stomach
Explanation of how the stomach produces hydrochloric acid without the lumen's cells being harmed in the process.
Mucosal Barrier
Once the hydrochloric acid is produced in the stomach lumen, this creates a low
(acidic) pH that could potentially destroy the cells in the mucosal layer of the
stomach. However, there are several structural and functional components that
are part of an effective mucosal barrier that protects the stomach cells and
prevents the stomach from digesting itself. The thick mucus layer produced by
gastric mucus cells has lots of bicarbonate ions that will buffer the effect of
the acid in the lumen. This stable, pH neutral mucus layer also provides a
barrier to the now activated pepsin enzymes in the lumen as well. The surface
gastric epithelial cells under the mucus layer have tight cell-to-cell junctions
that would prevent transcellular reabsorption of hydrochloric acid from the
lumen. Like epithelial tissue elsewhere in the body, these gastric surface cells
are replaced continually by gastric stem cells that are stimulated to divide
more rapidly if there is tissue damage from the gastric juice. Prostaglandins
are chemical messages involved in cell inflammation response to injury.
Prostaglandins produced in the stomach stimulate production of gastric mucus and
bicarbonate and promote tissue healing. When the mucosal barrier breaks down and
tissue destruction reaches into the deeper connective and muscular layers of the
stomach, this is called a peptic or gastric ulcer.
Example
Ulcers: When the Mucosal Barrier Breaks Down
Aspirin and other non-steroid inflammatory drugs (NSAIDs) are known to increase the
risk of gastric ulcers. NSAIDs break down the mucosal layer and reduce secretion of
bicarbonate ion, but even when they are enteric coated to protect the cells from
contact, they increase the risk of gastric ulcers.
The acidity of gastric juice destroys many, but not all bacteria. There is one
bacterium, Helicobacter pylori, adapted to live in the
stomach mucus layer. Most people have this bacterium in their stomach with no
symptoms, but in some people the bacterium is linked to increased incidence of
gastric ulcers. There is still uncertainty about why only some people are affected
this way. The bacterium uses the enzyme urease to convert urea to ammonia that helps
neutralize acid in the mucus layer locally. If the mucus becomes too acidic for the
bacterium, it can also induce an inflammatory response that lowers the gastric juice
acidity. This immune response and associate release of free radicals may stimulate
the formation of the ulcers in affected individuals. Once the causal link between
ulcers and H. pylori was discovered, a new treatment for ulcers was implemented and
the number of gastric ulcers in developed countries fell sharply.
There is evidence to suggest that the H. pylori should be considered to be colonizing
the stomach, like other GI tract microbiota, rather than infecting the stomach
because it does have some protective effects. As the incidence of gastric ulcers
fell in developed countries due to the use of antibiotics, the incidence of
esophageal reflux and cancer increased. Research has shown that the presence of H.
pylori is inversely related to the incidence of not only GERD and esophageal cancer,
but also childhood diarrhea, IBS, asthma and even tuberculosis. So there are
interesting interactions between this bacterium and its human host that need further
study.
Pancreatic Juice In Digestion
The pancreas produces about 1.2 to 1.5 quarts (1.1 – 1.4 liters) of
pancreatic juice each day. This clear, colorless liquid is
mostly water, along with some salts, sodium bicarbonate, and several digestive
enzymes. Sodium bicarbonate is responsible for the slight alkalinity of
pancreatic juice (pH 7.1 to 8.2). The sodium bicarbonate buffers the acidic
gastric juice which has arrived in the small intestine from the stomach,
inactivates pepsin from the stomach, and creates an optimal pH for the activity
of the digestive enzymes in the small intestine.
Just as the stomach produces pepsin in its inactive form (as pepsinogen), the
pancreas creates and produces its protein-digesting enzymes as zymogens. These
zymogens (trypsinogen, procarboxypeptidase, and chymotrypsinogen) are activated
in the duodenum. If produced in their active forms, they would digest the
pancreas itself. The intestinal enzyme enteropeptidase
convertstrypsinogen to the active trypsin. Trypsin alsoconverts
pancreatic enzymes procarboxypeptidase and chymotrypsinogen to their active
forms, carboxypeptidase and chymotrypsin. Some
pancreatic enzymes, including amylase, lipase, and
nuclease, are secreted in their active form, but their optimal
activity requires interactions with ions or bile in the intestinal lumen.
Bile
Bile is another accessory organ contribution to the GI tract lumen.
Bile is made by the hepatocytes of the liver, but stored in, concentrated, and
released from the gall bladder. Unlike pancreatic juice that is produced and
released as needed, bile is produced in small quantities by the liver
continuously. The gall bladder stores it for release into the small intestine as
needed.
Like saliva, bile is a mixture of mainly water with many substances, including
electrolytes, bile pigments, bile salts and lipids (including the
phospholipid lecithin and cholesterol).
The bile pigments include
bilirubin and biliverden, which are waste by-products from the
destruction of
the hemoglobin in aged or damaged red blood cells destroyed in the
liver. These
wastes are then eliminated from the body with the feces and
contribute to its
color. In addition, cells lining the bile duct secrete bicarbonate
ions into the bile. However, it is the lecithin and bile salts produced
from cholesterol that
have a digestive function related to dietary lipids.
 Emulsification of a fat globule by bile salts. This work by Cenveo is
licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Emulsification of a fat globule by bile salts. This work by Cenveo is
licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Recall that phospholipids
in the cell membrane have a hydrophilic and hydrophobic portion. Bile salts are
also amphiphilic. When they are released into the small intestine as part of the
bile, the hydrophobic/lipophilic portion of the molecule is attracted to fat
globules in the lumen, while the hydrophilic/lipophobic portion is attracted to
the water in the digestive juice mixture. This chemical attraction and
mechanical mixing breaks large fat globules into smaller droplets suspended in
the fluid. The bile salts prevent the droplets from rejoining and thus increase
the surface area of the lipid exposed to lipase enzymes; but the bile salts
don’t actually digest the lipid into fatty acids and monoglycerides. This
process is called emulsification.
Example
Emulsification
Think of what happens when you mix oil and vinegar for a salad dressing. You
can mechanically shake it up to produce smaller fat droplets suspended in
the vinegar, but if left to sit, the two layers separate again. Lecithin
from egg yolks is the emulsifier added to mayonnaise, which is a mixture of
oil in vinegar or lemon juice that has been emulsified.
Once the lipids have been digested, the bile salts are still attracted to the
lipid and form small micelles. These micells come in contact with the small
intestine cell membranes, releasing and facilitating absorption of its fatty
acids, monoglycerides and lipid-soluble vitamins. The bile salts are later
reabsorbed themselves in the large intestine and recycled through the hepatic
portal vein back to the hepatocytes, in what is called the enterohepatic
circulation. Soluble fiber in the diet, such as psyllium, interferes with the
reabsorption of these bile salts. As a result, the hepatocytes metabolize new
bile salts using cholesterol from the blood, helping to lower LDL cholesterol.
There are also other mechanisms by which both soluble and insoluble fiber affect
the body’s cholesterol metabolism and balance. The liver also excretes excess
cholesterol in the bile. When the bile becomes supersaturated with such excess
cholesterol, one of the three kinds of bile stones may form and obstruct the
bile ducts. Not surprisingly, this type of gallstone is often associated with
obesity. The absence of bile salts in bile results in a condition called
steattorhea, where fats remain mainly undigested in the feces and can lead to
diarrhea and fat malabsorption. This condition can result from liver damage or
be a side-effect after the gallbladder is removed (cholecystectomy).
Intestinal Juice
The intestinal glands at the base of villi produce intestinal juice, a mixture of
water and mucus. Each day, about one to two quarts (1-2 liters) are secreted.
The mucus in intestinal juice is produced by both the duodenal glands and the
goblet cells of the mucosa. The difference is that the mucus produced by the
duodenal glands is alkaline, while goblet cell mucus is typically neutral.
Progressing along the jejunum and into the ileum, goblet cells become more
abundant. Secretion of intestinal juice is primarily stimulated by distension of
the small intestine or the irritating effects of hypertonic or acidic chyme on
the intestinal mucosa. The mucus also protects the surface cells from abrasion
as the food moves through the lumen. Although the intestinal juice mucus
secreted doesn’t have a direct digestive function, it does play an important
role as a substrate for digestion. Extending into the mucus, but embedded in the
cell membrane of intestinal villi cells, are various enzymes. These cell
membrane surface enzymes are very important for the final digestion of organic
substances before absorption.
Carbohydrates in Digestion
The mouth is where the chemical digestion of starch and possibly glycogen begins.
Salivary amylase acts to break down the polysaccharide starch into the
disaccharide maltose, the trisaccharide maltotriose, and short chains of glucose
called α-dextrins.
Pancreatic amylase in the small intestine continues the chemical
digestion of starch and other digestible carbohydrates that have not been broken
down into maltose, maltotriose, and α-dextrins by salivary amylase. After starch
has been broken down into smaller fragments by salivary or pancreatic amylase,
the brush-border enzyme α-dextrinase starts working on the
resulting α-dextrins, breaking off one glucose unit at a time. The
disaccharides sucrose, lactose, and maltose are not digested until they enter
the small intestine. Here, three brush-border enzymes hydrolyze them into
monosaccharides. Sucrase splits sucrose into one molecule of
fructose and one molecule of glucose; lactase breaks down lactose
into one molecule of glucose and one molecule of galactose; and
maltase breaks down maltose and maltotriose into two and three
glucose molecules, respectively. The digestive system can then absorb
monosaccharides.
Proteins
The digestion of protein starts in the stomach, where pepsin breaks down proteins
into peptides. In the small intestine, trypsin and chymotrypsin hydrolyze
polypeptides into smaller peptides, and elastase fragments whole
proteins into peptides. The small peptides are then broken down into monomers.
Carboxypeptidase cleaves a single amino acid from the carboxy
terminus of a small peptide, whereas aminopeptidase cleave an amino
acid from the amino terminus. Dipeptidase splits dipeptides in the
middle, liberating two amino acids.
Lipids
Triglycerides and their breakdown products do not dissolve in water. Before they
can be digested in the watery environment of the small intestine, large lipid
globules must be separated into smaller lipid globules, a process called
emulsification, which is aided by the presence of bile salts. Recall that bile
salts facilitate emulsification of large lipid globules.intosmall lipid globules
of about 1 µm in diameter.[link to bile salts] This emulsification greatly
increases the surface area to volume ratio of fat globules, which allows
pancreatic lipase to access more lipid molecules.
The three lipases involved in the digestion of triglycerides and phospholipidsare lingual
lipase (in the saliva), gastric lipase, and pancreatic
lipase. Pancreatic lipase in the small intestine does most of the
lipid digestion. Pancreatic lipase breaks down triglycerides into fatty acids
and monoglycerides. The fatty acids include both short-chain (less than 10 to 12
carbons) and long-chain fatty acids.
Nucleic Acids
Nucleic acids, including DNA and RNA, are in the cells of all once-living things
which we may ingest. There are two nucleases in pancreatic juice –
deoxyribonuclease, which digests DNA, and
ribonuclease, which digests RNA. The nucleotides that are the
products of this digestion are further broken down by intestinal brush-border
enzymes (nucleosidases and phosphatases) into
pentosesugars, phosphates, and nitrogenous bases, which can be absorbed through
the GI tract wall.
The Digestive Enzymes
| Enzyme |
Source |
Food |
Product |
| Saliva |
| Lingual Lipase |
Lingual glands |
Triglycerides (fats and oils), other lipids |
Fatty acids and diglycerides |
| Salivary Amylase |
Salivary glands |
Polysaccharides (starches) |
α-Dextrins, disaccharide (maltose), trisaccharide (maltotriose) |
| Gastric juice |
| Gastric lipase |
Stomach chief cells |
Triglycerides (fats and oils) |
Fatty acids and monoglycerides |
| Pepsin* |
Stomach chief cells |
Proteins |
Peptides |
| Brush-border |
| α-Dextrinase |
Small intestine |
α-Dextrins |
Glucose |
| Enteropeptidase |
Small intestine |
Trypsinogen |
Trypsin |
| Lactase |
Small intestine |
Lactose |
Glucose and galactose |
| Maltase |
Small intestine |
Maltose |
Glucose |
| Nucleosidases and phosphatases |
Small intestine |
Nucleotides |
Phosphates, nitrogenous bases, and pentoses |
| Peptidases |
Small intestine |
Aminopeptidase: amino acid at amino end of peptides Dipeptidase:
dipeptides |
Aminopeptidase: amino acids and peptides Dipeptidase: amino acids |
| Sucrase |
Small intestine |
Sucrose |
Glucose and fructose |
| Pancreatic juice |
| Carboxy-peptidase* |
Pancreatic acinar cells |
Amino acid at carboxyl end of peptides |
Amino acids and peptides |
| Chymotrypsin* |
Pancreatic acinar cells |
Proteins |
Peptides |
| Elastase* |
Pancreatic acinar cells |
Proteins |
Peptides |
| Nucleases |
Pancreatic acinar cells |
Ribonuclease: ribonucleic acidDeoxyribonuclease: deoxyribonucleic
acid |
Nucleotides |
| Pancreatic amylase |
Pancreatic acinar cells |
Polysaccharides (starches) |
α-Dextrins, disaccharide (maltose), trisaccharide (maltotriose) |
| Pancreatic lipase |
Pancreatic acinar cells |
Triglycerides that have been emulsified by bile salts |
Fatty acids and monoglycerides |
| Trypsin* |
Pancreatic acinar cells |
Proteins |
Peptides |
*These enzymes have been activated by other substances.
A single layer of epithelial cells forms the inner most later of the stomach, called the
mucosa. The mucosa is marked by depressions called gastric pits (these are lined with
epithelial cells). The gastric gland is at the bottom of the pit, which contain multiple
cell types: mucous cells, stem cells, parietal cells, chief cells, and G cells
(enteroendocrine cells).
 Cells of the gastric pit in the mucosa. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Cells of the gastric pit in the mucosa. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Exocrine Function
Digestion in the stomach requires a combination of acid and pepsin. In the glands
of the stomach, chief cells secrete pepsinogen, which is converted
to pepsin, as well as gastric lipase, which is used for digestion of fats.
Parietal cells secrete hydrochloric acid (HCl) as well as
intrinsic factor.The gastric mucous cells associated with the surface and neck
of the gastric pit secrete a thick mucus that is more alkaline than the mucus
produced by goblet cells found elsewhere in epithelial tissue. This helps
provide a protective mucosal barrier from the acid and protein enzymes in the
gastric juice.
Endocrine Function
Regulation within the digestive system is tightly controlled by
enteroendocrine cells. Collectively, these cells are found in
the organs of the digestive system (entero- inside) and release hormones and
paracrine signals which regulate production of digestive chemicals and also
influence the nervous system for global regulation of digestion. Within the
stomach, the enteroendocrine cells are called G-cells.
G-cells secrete the signaling molecules including gastrin. Gastrin
signals parietal cells (HCl producing) and chief cells (enzyme producing) to
regulate production and alters stomach motility by altering the sphincter
function on either side of the stomach. G cells also secrete other paracrines –
serotonin, histamine, somatostatin, and other hormones which regulate
digestion.
Overview of Stomach Cells
| Structure |
Action |
Outcome |
| Gastric epithelial cells |
Structure of the stomach lumen |
Maintains structure of the mucosa |
| Chief cells |
Secrete pepsinogenSecrete gastric lipase |
Pepsin (activated form) breaks proteins down into peptidesBreaks down
triglycerides into fatty acids and monoglycerides |
| G cells |
Secrete gastrin |
Activates secretion of HCl by parietal cells and pepsinogen by chief
cells; contracts lower esophageal sphincter, enhances stomach motility,
and relaxes pyloric sphincter |
| Parietal cells |
Secrete HClSecrete intrinsic factor |
Kills microbes; denatures proteins, transforms pepsinogen into
pepsinEnables vitamin B12 absorption, which is necessary for red blood
cell formation |
Small Intestine Exocrine Function
The small intestine is primarily composed of simple columnar epithelial cells
with microvilli facing the lumen for absorption of digested material.
Intestinal crypts (also called the crypts of
Lieberkühn) are analogous to gastric glands of the stomach and are
formed by invaginations of the epithelium. Within the crypts are paneth
cells, which secrete multiple defensive proteins including lysozyme
definsins and phospholipase to protect the small intestine from pathogens which
have survived the stomach compartment. There are also Goblet cells,
which secrete mucous for protection from the acids of the stomach and enzymes
from accessory organs.
Small Intestine Endocrine Function
The small intestine also has enteroendocrine cells which secrete
hormones for regulation of absorption of nutrients. S cells produce
secretin to buffer intestinal pH. I cells (also called CCK
cells) secrete cholecystokinin, which stimulates the
release of digestive enzymes from the pancreas and gall bladder in to the
intestine. K cells secrete gastric inhibitory peptide
(GIP; also called glucose-dependent insulinotropic
peptide), which influences insulin levels
 100CX magnification of the small intestine epithelial cells showing
some of the densely packed microvilli. By Louisa Howard and Katherine
Connollly(Human jejunum microvilli). Public Domain. 100CX magnification of the small intestine epithelial cells showing
some of the densely packed microvilli. By Louisa Howard and Katherine
Connollly(Human jejunum microvilli). Public Domain.
|
 The lining of the small intestine. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/). The lining of the small intestine. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
|
| Cell Type |
Location in the Mucosa |
Function |
| Columnar epithelium with microvilli |
Lining |
Digestion and absorption of nutrients in chyme |
| Goblet |
Intestinal crypts |
Secretion of mucous |
| Paneth |
Intestinal crypts |
Secretion of the bactericidal enzyme lysozyme and other defensive proteins;
phagocytosis |
| Enteroendocrine |
|
|
| S cells |
Intestinal glands |
Secretion of the hormone secretin |
| I (or CCK) cells |
Intestinal glands |
Secretion of the hormone cholecystokinin |
| K cells |
Intestinal glands |
Secretion of the hormone gastric inhibitory peptide (GIP) |
Large Intestine Exocrine Function
Most of the mucosa of the large intestine is composed of simple columnar
epithelial cells. An exception is the distal anal canal, which is composed of
nonkeratinized stratified squamous epithelial cells. The stratified epithelium
is more durable to the abrasion that occurs when feces moves. The large
intestine also has crypts, which contain both epithelial cells and goblet cells.
Since most digestion and absorption occurs before the large intestine, the only
significant secretion is mucus, which lubricates the passage of digestive
residue.
Exocrine Function
Hepatocytes are the liver's main functional cells (hepato – liver,
cytes – cells). They play a role in a wide variety of secretory, metabolic, and
endocrine functions. Hepatocytes account for around 80 percent of the liver's
volume. Plates of hepatocytes called hepatic laminae (singular lamina) radiate
out from the center of hepatic lobules. Hepatocytes continually secrete bile,
but production and release accelerate when bile acid levels in the hepatic
portal blood (blood coming from the small intestine) increase. This means more
bile is secreted as digestion and absorption are proceeding in the small
intestine.
Immune Function
The liver also contains star-shaped Kupffer cells that are found in
the blood filled spaces (sinusoids) between laminae. These
phagocytic cells remove dead or damaged red and white blood cells, as well as
bacteria and other foreign material that potentially enter through the GI tract
and travel to the liver in the hepatic portal vein.
The pancreas is responsible for both endocrine (hormone secretion) and exocrine (enzyme
secretion) function. Cells are organized into clusters throughout the pancreas.
 Pancreas cellular structures, particularly beta cells that produce insulin.
This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Pancreas cellular structures, particularly beta cells that produce insulin.
This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Exocrine Function
The majority of the cells in the pancreas have an exocrine function are acini
cells. These epithelial cells were named this because they form clusters around
a small central space or lumen that connects to small ducts, looking much like a
cluster of grapes (acinus is Latin for berry or grape). Each acinus cell
(singular) has a wider basal side, toward the basement membrane, and a narrow
apical side toward the lumen, forming a ring or pie-shaped cells. They secrete
the various enzymes and bicarbonate ions that make up pancreatic juice into this
central duct.
Endocrine Function
The majority of the cells in the pancreas have an exocrine function are acini
cells. These epithelial cells were named this because they form clusters around
a small central space or lumen that connects to small ducts, looking much like a
cluster of grapes (acinus is Latin for berry or grape). Each acinus cell
(singular) has a wider basal side, toward the basement membrane, and a narrow
apical side toward the lumen, forming a ring or pie-shaped cells. They secrete
the various enzymes and bicarbonate ions that make up pancreatic juice into this
central duct.
You have already had some introduction to blood glucose homeostasis. We
will discuss the hormonal regulation of blood glucose further in the endocrine
unit. The pancreatic cells responsible for endocrine production are listed
below.
| Cell type |
Percent of islet cells |
Endocrine material |
Downstream effect |
| Alpha cells |
15-20% |
Glucagon |
Increases blood glucose of glycogen |
| Beta cells |
65-80% |
Insulin and amylin |
Lowers blood glucose |
| Delta cells |
3-10% |
Somatostatin |
Restricts absorption |
| PP cells |
3-5% |
Pancreatic polypeptide |
Regulates pancreatic function |
Throughout the gastrointestinal (GI) tract, walls are comprised of the same four
fundamental tissue layers. From the lumen of the GI tract, these layers are the mucosa,
submucosa, muscularis, and serosa.
 The lining of the small intestine. Image courtesy of Dr. Allan
Wiechmann, University of Oklahoma Health Sciences Center The lining of the small intestine. Image courtesy of Dr. Allan
Wiechmann, University of Oklahoma Health Sciences Center
|
 Tissue layers of the GI tract. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/). Tissue layers of the GI tract. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
|
The Mucosa
The mucosa is a mucous membrane that makes up the inner lining of the GI tract.
It has three layers: (1) the epithelium, made of closely packed cells without a
blood supply or nerves in direct contact with the foodstuffs that enter the GI
tract; (2) the lamina propria, a layer of connective tissue that supports the
epithelial cells; and (3) a thin smooth muscle layer called the muscularis
mucosae. In the mouth, pharynx, esophagus, and distal portion of the anal canal,
the epithelium is primarily nonkeratinized stratified squamous
epithelium. In the stomach and intestines, it is simple columnar epithelium,
whose cells participate in secretion and absorption. Every five to seven days,
the harsh chemical and mechanical environment of the GI tract causes epithelial
cells to be sloughed off and replaced by new ones. Epithelial cells are
interspersed with exocrine cells that secrete mucus and digestive fluid into the
lumen (interior space) of the alimentary canal and with
enteroendocrine cells that secrete hormones and paracrines. In addition to
connective tissue, the lamina propria of the mucosa contains
numerous blood and lymphatic vessels, which transport nutrients absorbed into
the GI tract to the liver. The lamina propria also contains most of the immune
cells that make up the mucosa-associated lymphatic tissue
(MALT).
The muscularis mucosae layer is not responsible for movement of
material through the GI tract, but it controls the exposed surface area. Small
alterations in the many small folds in the mucous membrane of the stomach and
small intestine increases the surface area available for digestion and
absorption. Of these folds, the rugae in the stomach are temporary structures
while the plicae circulares of the small intestine are permanent. When this
muscle layer contracts, rugae and plicae circulars are pushed together exposing
less SA to the lumen. When it relaxes more of the mucosal surface is revealed
and in contact with the digestive products in the lumen.
The Submucosa
The submucosa binds the mucosa to the muscularis externa. It is
composed of areolar connective tissue and includes blood and lymphatic vessels
(which transport absorbed food molecules) and the submucosal plexus (which is
part of nervous system control).
The Muscularis
In the mouth, pharynx, and superior and middle esophagus, the muscularis
externa contains skeletal muscle that we use for voluntary
swallowing. The external anal sphincter is also made of skeletal muscle, giving
us voluntary control of defecation. In the rest of the GI tract, the muscularis
externa is smooth muscle, which contracts involuntarily to break down food, mix
it with digestive juices, and move it along the GI tract. Complementary muscles
in longitudinal (along the length of the tract) and circular layers creates
peristalsis – the wave-like muscular movements to move good from the esophagus
to the anus.
The Serosa
The serosa is the superficial layer of the intestine that covers the
parts of the GI tract that is exposed to the abdominal cavity. This serous
membrane is made up of areolar connective tissue and simple squamous epithelium
(mesothelium). The esophagus has a single layer of tough
connective tissue called the adventitia; it does not have a
serosa.
The peritoneum, is the largest serous membrane in the body and
lines the abdominal cavity. The tissue has several components including the the
mesothelium and an underlying supporting areolar connective tissue layer. The connective
tissue in turn has two layers: the parietal peritoneum, which
lines the abdominal wall, and the visceral peritoneum, which
covers some organs and serves as their serosae. The peritoneal cavity
is the small space between the parietal and visceral peritoneum that contains the
lubricating serous fluid.
The peritoneum includes large folds that bind organs to each other and to the abdominal
walls. This keeps the organs in the proper place and suspends them when we are upright.
Within these folds are blood vessels, lymphatic vessels, and nerves that innervate the
abdominal organs.
The Five Major Peritoneal Folds
| Fold |
Description |
Comment |
| Greater omentum |
Drapes over the transverse colon and small intestine |
High adipose tissue content vastly expands with weight gain, creating
the characteristic "beer belly" |
| Falciform ligament |
Attaches the liver to the anterior abdominal wall and diaphragm |
The liver is the only digestive organ attached to the anterior abdominal
wall |
| Lesser omentum |
Suspends the stomach and duodenum from the liver |
Provides a pathway for the blood supply of liver; contains the common
bile duct |
| Mesentery |
Attaches the jejunum and ileum of the small intestine to the posterior
abdominal wall |
Includes blood and lymphatic vessels, and lymph nodes |
| Mesocolon |
Attaches the transverse colon and sigmoid colon of the large intestine
to the posterior abdominal wall |
Along with mesentery, it holds intestines loosely in place, enabling
movement with muscle contractions |
Example
Homeostatic Imbalances of the Peritoneum: Peritonitis
Inflammation of the peritoneum is called peritonitis. It can be caused by an
injury that penetrates into the abdomen or from an ulcer that perforates the
stomach wall, allowing gastric fluids into the peritoneal cavity. The most
common cause of peritonitis is a ruptured appendix. The appendix is a
terminal part of the cecum (a peritoneal pouch at the beginning of the large
intestine), and the function (if any) is still debated. When the appendix
bursts open, bacteria-laden feces spurt into the peritoneum. Usually, the
peritoneal layers will bind together around the site of inflammation,
keeping the infection from spreading, while macrophages move in to dispose
of infected tissue. Peritonitis that spreads out into the peritoneal cavity
can be life-threatening. The condition is treated by surgically removing the
infected tissue and administering high doses of antibiotics. Generally,
peritonitis is a concern with any kind of puncture wound to the abdomen.
The cheeks, tongue, hard palate, and soft palate frame the mouth, also called the
oral cavity or buccal cavity. The mouth is involved in
both mechanical and chemical digestion. Mechanical digestion consists of
mastication (chewing), in which the tongue manipulates food, the teeth
grind it, and saliva mixes with it. Mastication turns food into an easy-to-swallow bolus
and breaks the food into smaller pieces so that there is more contact area for digestive
enzymes.
The cheeks make up the oral cavity's lateral walls. The outer covering of the cheeks is
the skin, and the inner covering is the mucous membrane. This membrane is made up of
nonkeratinized stratified squamous epithelium; the multiple layers are resistant to
abrasion. Between the skin and mucous membranes are connective tissue, fat tissue and
buccinator muscles.
 The bounds of the oral cavity. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The bounds of the oral cavity. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The lips, also called labia ("fleshy borders"), encircle the opening of the mouth. Their
outer covering is skin, and their inner lining is mucous membrane. Between these two
layers is the orbicularis oris muscle. The labial frenulum is a midline
fold of mucous membrane that attaches the inner surface of each lip to the gum. When we
chew food, the buccinator muscles in the cheeks and the orbicularis oris muscle in the
lips contract, which keeps food between the lower and upper teeth. These muscles also
play a role in speech.
The area between the lips (or cheeks) and teeth is called the oral
vestibule. The oral cavity is the area than runs between the gums
and teeth and the entrance to the throat (oropharynx), also called the
fauces ("passages"). If you puff out your cheeks, you can increase the
size of the oral vestibule but not the oral cavity.
The septum that separates the oral cavity and nasal cavity is called the
palate. Anatomically, a septum (plural, septa) is a wall within a single organ or cavity
that separates the space into distinct sides. For example, the nasal septum divides the
nostrils. This is separating two distinct cavities.
 Structures of the oral cavity. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Structures of the oral cavity. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The palate, which forms the roof of the mouth, is what allows us to breathe
while chewing food. The anterior part of the roof of the mouth is called the hard
palate. It is created by the maxillae and palatine bones and is covered by
mucous membrane. The hard palate makes up the bony wall between the oral and nasal
cavities. The posterior part of the roof of the mouth, the soft palate, is
also lined with mucous membrane. This arch-shaped muscular structure forms a dividing
wall between the oropharynx and nasopharynx.
The uvula (Latin for ‘little grape’) is a cone-shaped muscular process that
hangs from the end of the soft palate. It plays a role in speech and articulation of
words. It also plays a small role in preventing foods and liquids from entering the
nasal cavity. Two muscular folds on each side of the base of the uvula extend down the
lateral sides of the soft palate. The anterior fold is called the palatoglossal
arch, which terminates next to the base of the tongue. The posterior fold,
the palatopharyngeal arch, lies at the interface with the pharynx. Between
these two arches on the lateral wall are the palatine tonsils. The
lingual tonsils are located at the base of the tongue.
There are also many small, intrinsic salivary glands within the mucous membrane of the
mouth and tongue. Their secretion is independent of the presence of food. These glands
either open directly into the oral cavity or indirectly through short ducts. Small
amounts of saliva are secreted by the labial glands in the lips, the
buccal glands in the cheeks, the palatal glands in the
palate, and the lingual glands in the tongue.
The tongue is composed of skeletal muscle covered with a surface of nonkeratinized
stratified squamous epithelium and a mucous membrane covering. The tongue and its
associated muscles make up the floor of the oral cavity. A median septum extends the
entire length of the tongue, dividing it into symmetrical halves. Inferiorly, the tongue
is attached to the mandible, styloid process of the temporal bone, and hyoid bone.
 The tongue. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The tongue. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Each half of the tongue has the same number and type of intrinsic and extrinsic muscles.
As you learned in your studies of the muscular system, extrinsic muscles of the tongue are the hyoglossus, genioglossus, styloglossus
and palatoglossus muscles. These muscles originate outside the tongue and
insert into connective tissues within the tongue. They move the tongue from side to side
and in and out, performing three important digestive functions: (1) moving food for
optimal chewing, (2) shaping food into a bolus (rounded mass), (3)
pushing food toward the back of the mouth to be swallowed and (4) elevates the posterior
part of the tongue to assist in swallowing. Conscious control of the tongue also aids in
speaking and other non-digestive processes.
The intrinsic muscles are the longitudinalis superior, longitudinalis inferior, transversus
linguae, and verticalis linguae muscles (lingua and
lingual refers to the tongue). These muscles both originate in and insert into
connective tissues within the tongue. They change the size and shape of the tongue to
facilitate swallowing and speech. A fold of mucous membrane in the middle inferior
region (underside) of the tongue, the lingual frenulum, attaches
to the floor of the mouth and limits the tongue's posterior movement. People with a
condition called ankyloglossia have a too short or too rigid
lingual frenulum, which impairs their speech. As a result, they are sometimes referred
to as "tongue-tied."
The top and sides of the tongue are studded with papillae
("nipple-shaped") extensions of lamina propria layer of mucosa, which are enshrouded in
stratified squamous epithelium. Most of these papillae contain taste buds; those without
taste buds have touch receptors to give a sense of food’s texture. The latter increase
the amount of friction between the tongue and food, which helps the tongue move food
around in the mouth. Lingual glands, are found on the anterior, inferior surface of the
tongue, in the lamina propria of the tongue secrete mucus and a watery serous fluid that
contains the enzyme lingual lipase, which begins the digestion of
triglycerides.
 Specific teeth and structures of the mouth. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Specific teeth and structures of the mouth. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The teeth, or dentes, are secured in sockets of the alveolar processes of
the maxillae and mandible. Gingivae (gums; singular gingiva) cover the
alveolar processes. Lining the sockets is the periodontal ligament, a dense
fibrous connective tissue that secures the teeth in place. The teeth are covered by
enamel, which is the hardest substance in the body. Enamel helps
prevent teeth from being worn down when we chew, and it helps keep out acids that could
easily dissolve the interior of a tooth.
Humans have two sets of teeth (dentitions). The 20 deciduous teeth, or baby teeth, first appear at about 6 months of age. Between
ages 6 and 12 years, these teeth are replaced by the 32 permanent teeth.
Closest to the midline are the two incisors, chisel-shaped teeth we use for
cutting into food. The cuspids (canines) are next to
the incisors. A cuspid’s pointed edge (cusp) tears and shreds
food. Posteriorly, the next teeth are the two premolars (bicuspids) followed by up to
three molars. Premolars and molars have broader, flatter surfaces, which we use to crush
and grind food.
| Structure |
Action |
Outcome |
| Lips and cheeks |
Confine food between teeth |
Foods are chewed evenly during mastication |
| Salivary glands |
Secrete saliva |
Moistens and lubricates the lining of mouth and pharynxMoistens,
softens, and dissolves food so we can tasteCleans mouth and
teethSalivary amylase breaks down starch |
| Tongue - Extrinsic muscles |
Tongue moves sideways, in and out, and elevates posteriorly |
Maneuvers food for chewingShapes food into bolusManeuvers food for deglutition (swallowing) |
| Tongue - Intrinsic muscles |
Tongue changes shape |
Maneuvers food for deglutition (swallowing) |
| Tongue - Taste buds |
Sense taste and presence of food in mouth |
Nerve impulses from taste buds are conducted to salivatory
nuclei in brain stem and then to salivary glands, stimulating saliva
secretion |
| Lingual glands |
Secrete lingual lipase |
Breaks down triglycerides into fatty acids and diglycerides |
| Teeth |
Cut, tear, and crush food |
Breaks down solid food into smaller particles for deglutition (swallowing) |
The pharynx (throat) is a funnel-shaped tube that runs from the
choanae (internal nostrils) to the esophagus posteriorly and to the larynx anteriorly.
The pharynx has three subdivisions: the nasopharynx, the oropharynx, and the
laryngopharynx. The nasopharynx acts as a passageway for air
during ventilation, while the other two subdivisions participate in both ventilation and
digestion. Superiorly, the oropharynx is connected with the nasopharynx, inferiorly with
the larynx and laryngopharynx, and anteriorly with the mouth.
 The Pharynx. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The Pharynx. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Histologically, the wall of the pharynx is similar to that of the oral cavity. The mucosa
includes a epithelium with mucous-producing glands. The nasopharynx is lined with
pseudostratified columnar epithelium; the oropharynx and laryngopharynx are lined with
stratified squamous. There are two skeletal muscle layers in the external muscle layer
of the pharyngeal wall. Cells in the inner layer extend longitudinally. In the outer
layer, the superior, middle, and inferior pharyngeal constrictor muscles are stacked, one inside
the other, like flowerpots. Contraction of these muscles propels food toward the
esophagus.
When swallowed food enters the oropharynx and laryngopharynx, it provokes contractions of the pharyngeal constrictor muscles
that help push food into the esophagus. Usually during swallowing, the soft palate moves
back to close off the nasopharynx, while the trachea ("windpipe") moves up under the
epiglottis to cover the glottis (the opening to the larynx or voice box); this
effectively blocks off air passages. But we all know what it feels like to have food "go
down the wrong tube," which means it either goes into the nasal cavities or the trachea.
When food enters the trachea, our reaction is to cough, which usually forces the food up
and out of the trachea and back into the pharynx.
 The Esophagus. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The Esophagus. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The esophagus is a collapsible tube located posterior to the trachea. This
muscular tube is about 10 inches long. The esophagus runs a mainly straight route
through the mediastinum of the thorax. To enter the abdomen, the esophagus penetrates
the diaphragm through an opening called the esophageal hiatus. At the
cardiac orifice, the esophagus joins with the stomach. Surrounding this
orifice is the lower esophageal sphincter (also called the
gastroesophageal or cardiac sphincter). Remember that
sphincters are circular muscles that surround tubes and serve as valves, closing the
tube when the sphincters contract and opening it when they relax. The lower esophageal
sphincter relaxes to let food pass into the stomach, then contracts to prevent stomach
contents from backing up into the esophagus. Surrounding this sphincter is the muscular
diaphragm, which helps close off the sphincter when no food is being swallowed. When the
lower esophageal sphincter does not completely close, some of the stomach contents can
reflux (i.e., back up into the esophagus), causing heartburn or gastroesophageal reflux
disease (GERD).
The esophageal mucosa is made up of nonkeratinized stratified squamous epithelium, lamina
propria, and a muscularis mucosa. The mucosa near the stomach also includes mucous
glands. The stratified squamous epithelium protects against abrasion and erosion from
food particles. In the superior third of the esophagus, the muscularis is skeletal
muscle, in the middle third it is both skeletal and smooth muscle, and in the inferior
third it is smooth muscle. A slight prominence at either end of the esophagus creates
two sphincters: the upper esophageal sphincter, made of skeletal muscle,
and the lower esophageal sphincter, made of smooth muscle. As we have already learned,
the superficial layer of the esophagus is called the adventitia, not the serosa. In
contrast to the stomach and intestines, the areolar connective tissue of the adventitia
is not covered by mesothelium.
The esophagus secretes mucus that lubricates food, and it propels food into the stomach.
Chemical digestion that began in the mouth continues in the esophagus, but there aren’t
any new digestive enzymes secreted. The upper esophageal sphincter controls the movement
of food from the pharynx into the esophagus. The lower esophageal sphincter controls the
movement of food from the esophagus into the stomach. Rhythmic waves of peristalsis
begin in the esophagus and then continue in the rest of the digestive tract organs. The
feeling of having a lump in your throat when you are nervous is caused by peristalsis
that occurs when there is no food in the esophagus.
| Action |
Outcome |
| Upper esophageal sphincter relaxation |
Allows bolus to move from the laryngopharynx to the esophagus |
| Peristalsis |
Propels bolus down the esophagus |
| Lower esophageal sphincter relaxation |
Allows bolus from the esophagus to enter the stomach |
| Mucus secretion |
Lubricates the esophagus, allowing easy passage of bolus |
Deglutition is another word for swallowing – the movement of food
from the mouth and into the stomach. The entire process takes about four to eight
seconds for solid or semisolid food and about one second for very soft food and liquids.
Deglutition involves the mouth, pharynx, and esophagus. It is facilitated by the
secretion of mucus and saliva. There are three stages in deglutition: the voluntary
phase, the pharyngeal phase, and the esophageal phase.
 Bolus position during deglutition. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Bolus position during deglutition. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The voluntary phase of deglutition is also called the oral phase or buccal phase. In this phase,
swallowing is set in motion when the tongue moves upward and backward against the
palate, pushing the bolus to the back of the oral cavity and into the oropharynx. At
this point, the two involuntary phases of swallowing begin.
In the pharyngeal phase, stimulation of receptors in the oropharynx
sends impulses to the deglutition center (a collection of neurons
that controls swallowing) in the medulla oblongata. Impulses are then sent back to the
uvula and soft palate, provoking them to move upward to close off the nasopharynx.
Sequential contractions of the pharyngeal constrictor muscles move the bolus through the
oropharynx and laryngopharynx. Relaxation of the upper esophageal sphincter then allows
food to enter the esophagus.
The entry of food into the esophagus marks the beginning of the esophageal phase of deglutition. Peristalsis propels the bolus through the
esophagus and toward the stomach. The circular muscle layer of the muscularis
immediately superior to the bolus contracts, pinching the esophageal wall, forcing the
bolus forward. At the same time, the longitudinal muscle layer of the muscularis
inferior to the bolus also contracts, shortening this inferior area and pushing out its
walls to receive the bolus. Waves of contractions keep moving the food toward the
stomach. When the bolus nears the stomach, relaxation of the lower esophageal sphincter
allows the bolus to pass into the stomach. During the esophageal phase, esophageal
glands secrete mucus that lubricates the bolus and minimizes friction. Peristalsis is
completely involuntary even though there is skeletal muscle in the first 2/3 of the
esophagus.
The stomach is an expansion of the GI tract that lies below the esophagus. It is a very
mobile structure between its relatively fixed upper and lower ends. The stomach is found
between the esophagus to the first part of the small intestine (the duodenum). The
stomach's position and size are always changing. It is about as big as a large sausage
when empty, and stretches to hold 1 liter of food when full; the stomach has a final
capacity of 2-3 liters. One of the digestive functions of the stomach is to serve as a
temporary "holding chamber". This is an important step in digestion, because we can eat
a meal far more quickly than it can be digested and absorbed in the small intestine. So
the stomach pushes only a small amount of food into the small intestine at a time.
The stomach plays several important roles in chemical digestion, including the continued
digestion of starch and the initial digestion of proteins and triglycerides. The stomach
is also where the semisolid bolus is converted to a liquid (chyme) and where some
ingested substances are absorbed.
Stomach Anatomy
There are four main regions in the stomach: the cardiac, fundus, body, and
pylorus. The cardiac (cardia region)
is a small area surrounding the cardiac orifice through which food from the
esophagus enters the stomach. Lying beneath the diaphragm, superior and to the
left of the cardiac, is the dome-shaped fundus, which
functions as a temporary storage center for food. Inferior to the fundus is the
body, the large central part of the stomach. The
funnel-shaped pylorus connects the stomach to the
duodenum. The pyloric antrum is the wider, more superior
portion of the pylorus that connects to the body of the stomach. The pyloric
antrum narrows into a region called the pyloric canal,
which leads into the duodenum. The pylorus communicates with the duodenum via
the smooth muscle pyloric sphincter. This sphincter
controls stomach emptying. In the absence of food, the stomach deflates inward
and its mucosa and submucosa fall into large folds called rugae.
The convex lateral surface of the stomach is called the greater
curvature; the concave medial border is the lesser
curvature. Two fatty mesenteries (peritoneal folds) called omenta hold
the stomach in place. The lesser omentum extends from the liver to the lesser
curvature. The greater omentum runs from the greater curvature to the posterior
abdominal wall.
Adaptations of Stomach Wall
The wall of the stomach is made of the same four tissue layers as most of the
rest of the GI tract, but with adaptations to the mucosa and muscularis externa
for the unique functions of this organ. In addition to the typical circular and
longitudinal smooth muscle layers, the muscularis externa has an inner oblique
smooth muscle layer that runs diagonally around the organ. As a result, in
addition to moving food through the GI tract, the stomach can more vigorously
churn and mix food, mechanically breaking it down into smaller particles.
 Gatric pit. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/). Gatric pit. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
|
 Histology section of a gastric pit. This work is licensed under a
Creative Commons Attribution-Noncommercial-Share Alike 3.0 License©
copyright 2010 Regents of the University of Michigan.
Histology section of a gastric pit. This work is licensed under a
Creative Commons Attribution-Noncommercial-Share Alike 3.0 License©
copyright 2010 Regents of the University of Michigan.
|
Millions of deep gastric pits in the epithelium lead into gastric glands, which secrete gastric juice.
The walls of the gastric pits are made up primarily of epithelial cells. The gastric
glands are made up of different types of cells. Glands of the cardia and pylorus are
composed primarily of mucus-secreting cells. Cells that make up the pyloric antrum
secrete mucus and a number of hormones, including gastrin. The much larger glands of the
fundus and body of the stomach, the site of most chemical digestion, produce most of the
gastric secretions. These glands are made up of a variety of secretory cells, including
mucous neck, parietal, chief, and enteroendocrine cells (G-cells).
| Structure |
Action |
Outcome |
| Muscularis Externa |
Mixing wavesPeristalsis |
Forms chyme by liquefying food and mixing it with gastric juicePropels chyme
through pyloric sphincter |
| Pyloric sphincter |
Controls passage of chyme from the stomach to the duodenum |
Delivers chyme to the intestine at a rate optimal for digestion and absorption;
prevents chyme from flowing back to stomach from duodenum |
| Chief cells |
Secrete pepsinogenSecrete gastric lipase |
Pepsin (activated form) breaks proteins down into peptidesBreaks down
triglycerides into fatty acids and monoglycerides |
| G cells |
Secrete gastrin |
Activates secretion of HCl by parietal cells and pepsinogen by chief cells;
contracts lower esophageal sphincter, enhances stomach motility, and relaxes
pyloric sphincter |
| Parietal cells |
Secrete HClSecrete intrinsic factor |
Kills food microbes; denatures proteins, transforms pepsinogen into
pepsinEnables vitamin B12 absorption for red blood cell formation |
| Surface mucous cells and mucous neck cells |
Secrete mucusAbsorption |
Protects stomach wall from digestive processesAllows limited amount of water,
ions, short-chain fatty acids, and certain drugs to enter bloodstream |
The secretion of gastric juice is controlled by nerves, hormones, and the presence of
products of digestion. Stimuli from smell, sight and taste as well as food in the oral
cavity, stomach, and small intestine activate or inhibit gastric juice production. This
is why the three phases of gastric secretion are called the cephalic, gastric, and
intestinal phases. However, once gastric secretion begins, all three phases can occur
simultaneously.
The cephalic (reflex) phase of gastric secretion,
which lasts just a few minutes, takes place before food enters the stomach. The smell,
taste, sight, or thought of food triggers this phase. For example, impulses from
receptors in taste buds or the nose are relayed to the brain, which returns signals that
increase gastric secretion to prepare the stomach for digestion. This enhanced secretion
is a conditioned reflex, meaning it occurs only if we like or
want a particular food. Depression and loss of appetite can suppress the cephalic
reflex.
The gastric phase of secretion lasts three to four hours and is set in
motion by local neural and hormonal mechanisms triggered by the entry of food into the
stomach. For example, stomach distension activates stretch receptors that stimulate
parasympathetic neurons to release acetylcholine, which then provokes increased
secretion of gastric juice. Partially digested proteins, caffeine, and rising pH
stimulate the release of gastrin from enteroendocrine G cells, which in
turn provokes parietal cells to increase their production of HCl and increase motility
through the stomach by altering sphincter function. The presence of proteins in the
stomach raises pH, which stimulates the increased gastrin and HCl release needed to
create an acidic environment for protein digestion.
Example
GERD
If this control of gastric juice secretion is not working properly and too much
hydrochloric acid is produced, conditions such as Gastroesophageal Reflux Disease
(GERD) can occur. The acidic pH irritates the lining of the esophagus near the
cardiac or lower esophageal sphincter and can lead to esophageal cancer. Antacids
provide only temporary relief. Some drugs known as histamine-2 antagonists
(H2-blockers) or proton-pump inhibitors (PPIs) may be needed to control the amount
of stomach acid produced. However, the two drugs act on different parts of the
parietal cell metabolism.
Review the process of hydrochloric acid secretion by parietal cell to analyze the physiology behind these two
drugs.
The stomach participates in virtually all digestive activities with the exception of
ingestion and defecation. In addition to mechanical and chemical digestion, the stomach
absorbs some fat-soluble substances, including alcohol and aspirin.
Mechanical Digestion
A few minutes after food enters the stomach, mixing waves begin at intervals of
about 15 to 25 seconds. Mixing waves are a gentle type of
peristalsis that soften and moisten food. They also combine food with gastric
juice and create chyme. Initial gentle mixing waves are followed by more intense
waves that break down food into smaller pieces and further mix with digestive
juice, starting at the body of the stomach and increasing in force as they reach
the pylorus.
The pylorus, which holds around 30 mL (1 ounce) of chyme, acts as a filter,
permitting only liquids and small food particles to pass through the mostly, but
not fully, closed pyloric sphincter. When food enters the pylorus, periodic
mixing waves force about 3 mL of chyme through the pyloric sphincter and into
the duodenum, a process called gastric emptying. The rest
of the chyme is pushed back into the body of the stomach, where it continues
mixing. This process is repeated when the next mixing waves force more chyme
into the duodenum. These forward and backward movements accomplish most of the
mixing in the stomach.
Gastric emptying is regulated by both the stomach and the duodenum. The presence
of chyme in the duodenum activates receptors that inhibit gastric emptying. This
prevents additional chyme from being released by the stomach before the duodenum
is ready to process it.
Chemical Digestion
Some foods sit in the fundus of the stomach for an hour or so before they are
mixed with gastric juice. During this time, the digestive activity of salivary
amylase continues. Mixing waves soon take over however, combining chyme with
acidic gastric juice, which inactivates salivary amylase and activates lingual
and gastric lipase. Lingual lipase then starts breaking down triglycerides into
fatty acids and diglycerides.
The digestion of protein begins in the stomach, primarily by pepsin. During
infancy, gastric glands also produce rennin, an enzyme
that helps digest milk protein. Lingual lipase secreted by the salivary glands
may also participate in triglyceride digestion in the stomach. Parietal cells
secrete hydrogen ions and chloride ions into the stomach lumen, effectively
forming HCl, which denatures dietary proteins.
The contents of the stomach are completely emptied into the duodenum within two
to four hours after you eat a meal. Carbohydrate-rich foods are the quickest to
leave the stomach. High-protein foods exit a little more slowly. Fatty meals
with high triglyceride content remain in the stomach the longest. Enzymes in the
small intestine digest fats slowly. So when the duodenum is processing fatty
chyme, food can stay in the stomach for six hours or longer.
The small intestine is a convoluted tube that begins just distal to the pyloric sphincter
of the stomach and then loops through the central and inferior region of the abdomen
before ending at the ileocecal valve, where it merges with the large intestine. The
small intestine is the primary digestive organ in the body. Not only is it the part of
the GI tract where digestion is completed, it is also where the majority of absorption
occurs.
Although the small intestine is the longest part of the GI tract, its diameter is about
half that of the large intestine, averaging a little over one inch (2.5 cm). When we are
alive, the small intestine is more than 3 meters (10 feet) long – the size of a
one-story building. Its length provides expansive surface area necessary for digestion
and absorption. However, circular folds, villi, and microvilli add even more surface
area. With loss of muscle tone after death, the folds of the small intestine relax,
extending it to about 20 feet in length.
 This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Small Intestine Anatomy
The duodenum precedes the jejunum and ileum and is the shortest part
of the small intestine; it is less than 1 foot of the 10 foot intestine (30 cm
of the 3 m). The duodenum receives the stomach contents, pancreatic juice and
bile. Chemical digestion continues in the duodenum. The jejunum is
the next portion (3.5 - 5.5 ft or 110-170 cm) of the small intestine and is
responsible for the absorption of a majority of nutrients. In the last section
of the small intestine, the ileum, vitamin B12 and bile salts are
absorbed as well as materials not absorbed by the jejunum.
The term mucosa or mucous membrane always refers to the combination
of the epithelium plus the lamina propria. The lamina propria layer of the small
intestinal mucosa is composed of areolar connective tissue and quite a bit of
mucosa-associated lymphoid tissue. Most solitary lymphatic nodules are located
in the distal portion of the ileum. The blood supply which runs through the
lamina propria and drains the villus is part of the the hepatic portal systems
and not general systemic circulaions
In the ileum, the lamina propria also has aggregated lymphatic
follicles (groups of lymphatic nodules) called Peyer's
patches. There are more Peyer's patches toward the end of the small
intestine, possibly because of the high amounts of bacteria in that area must be
prevented from entering the bloodstream. The submucosa of the duodenum is the
only site of the complex mucus-secreting duodenal (Brunner's)
glands, which produce a bicarbonate-rich alkaline mucus that helps
buffer the acidic chyme coming in from the stomach.
Like in the rest of the GI tract, the muscularis externa layer is made of two
layers of smooth muscle – an outer, thinner layer of longitudinal fibers and an
inner, thicker layer of circular fibers. The serosa completely enshrouds the
small intestine, with the exception of a large region of the duodenum.
Each day, the alimentary canal processes up to 10 liters of food, liquids, and GI
secretions; of which, only about one liter enters the large intestine. Almost all
ingested food and drink, 80 percent of electrolytes, and most of the water are absorbed
in the small intestine. The entire small intestine is involved in absorption, but most
absorption occurs before chyme reaches the ileum. Absorption in the ileum
primarily involves the recycling of bile salts. The absorptive capacity of
the alimentary canal is amazing. By the time chyme passes from the ileum into the large
intestine, it is essentially indigestible food residue (mainly plant fibers like
cellulose) some water, and millions of bacteria.
The absorption of most nutrients through the mucosa of the intestinal villi
occurs via active transport mechanisms that are driven directly or indirectly by
metabolic energy. These nutrients enter the capillary blood in the villus and travel to
the liver via the hepatic portal vein. An exception is certain lipids,
which undergo passive absorption via diffusion and then enter the modified lymph duct in
the villus (called a lacteal), to be transported to the blood in lymphatic
fluid. Substances cannot be absorbed between the epithelial cells of the
intestinal mucosa, because these cells connect with tight junctions. This is why
substances can only enter blood capillaries by passing through the epithelial cells and
into the interstitial fluid.
Carbohydrate Absorption
All carbohydrates are absorbed in the form of monosaccharides. The small
intestine is highly efficient at this, absorbing monosaccharides at an estimated
rate of 120 grams per hour. All normally digested dietary carbohydrates are
absorbed. However, indigestible fibers including cellulose, glucose polymers
made by plants which is not broken down in humans, are eliminated in feces. The
monosaccharides glucose and galactose – the products of the breakdown of starch
and disaccharides – are transported into the epithelial cells by common protein
carriers via secondary active transport, which requires ATP). They leave these
cells via facilitated diffusion and enter the capillaries through intercellular
clefts. The monosaccharide fructose (which is in fruit) is absorbed and
transported by facilitated diffusion alone. Monosaccharides combine with the
transport proteins, which lie very near the disaccharide splitting enzymes on
the microvilli, immediately after disaccharides are broken down.
Protein Absorption
Active transport mechanisms, primarily in the duodenum and jejunum, absorb most
proteins as their breakdown products, amino acids. Almost all (95 to 98 percent)
protein is digested and absorbed in the small intestine. The type of carrier
that transports an amino acid varies. Most carriers are linked to the active
transport of sodium. Short chains of two amino acids (dipeptides) or three amino
acids (tripeptides) are also transported actively. But after they enter the
absorptive epithelial cells, they are broken down to their amino acids before
leaving the cell and entering the capillary blood via diffusion.
Lipid Absorption
Despite being hydrophobic, the small size of short-chain fatty acids enables them
to be absorbed in intestinal chyme, enter absorptive cells via simple diffusion,
and then take the same path as monosaccharides and amino acids into the blood
capillary of a villus. The large and hydrophobic long-chain fatty acids and
monoglycerides are not so easily suspended in the watery intestinal chyme.
However, bile salts and the phospholipid lecithin (also found in bile) help make
them more soluble by surrounding them and creating tiny spheres called micelles.
Micelles are macromolecular structures made up of
aggregates of fatty elements and bile salts that create a polar (hydrophilic)
end that faces the water and a nonpolar (hydrophobic) core. The core also
includes cholesterol molecules and fat-soluble vitamins. Micelles can easily
squeeze between microvilli and get very near the luminal cell surface. At this
point, lipid substances exit the micelle and are absorbed via simple diffusion.
Without their "vehicles" (i.e., the micelles), lipids would sit on the surface
of chyme, like oil on water, and would never come in contact with the absorptive
surfaces of the epithelial cells. About 95 percent of lipids are absorbed in the
small intestine. Bile salts not only speed up lipid digestion, they are also
essential to the absorption of the end products of lipid digestion.
The free fatty acids and monoglycerides that enter the epithelial cells are
reincorporated into triglycerides, which are then mixed with phospholipids and
cholesterol and surrounded with a protein coat, creating chylomicrons. Chylomicrons are milky-white droplets of water-soluble
lipoprotein that are processed by the Golgi apparatus for release from the cell.
Chylomicrons are too big to pass through the basement membranes of blood
capillaries. Instead, they enter the much larger pores of lacteals. This means
that most fats are first transported in the lymphatic vessels and do not enter
the venous blood until they reach the thoracic duct in the neck region. In the
bloodstream, the enzyme lipoprotein lipase breaks down the
triglycerides of the chylomicrons into free fatty acids and glycerol. These two
breakdown products can then pass through capillary walls to become energy used
by our cells or to be stored in adipose tissue as fats. Liver cells combine the
remaining chylomicron material with proteins, forming "new" lipoproteins that
transport cholesterol in the blood.
Nucleic Acid Absorption
The products of nucleic acid digestion – pentose sugars, nitrogenous bases, and
phosphate ions –are transported across the epithelium by special carriers in the
villus epithelium via active transport.
| Food/Breakdown Products |
Absorption Mechanism |
Entry to Bloodstream |
Destination |
| Carbohydrates |
| Glucose |
Co-transport with sodium ions |
Capillary blood in villi |
Liver via hepatic portal vein |
| Galactose |
Co-transport with sodium ions |
Capillary blood in villi |
Liver via hepatic portal vein |
| Fructose |
Facilitated diffusion |
Capillary blood in villi |
Liver via hepatic portal vein |
| Protein |
| Amino acids |
Co-transport with sodium ions |
Capillary blood in villi |
Liver via hepatic portal vein |
| Lipids |
| Long-chainfatty acids |
Diffusion into intestinal cells, where they are combined with proteins to create
chylomicrons |
Lacteals of villi |
Systemic circulation via lymph in thoracic duct |
| Monoglycerides |
Diffusion into intestinal cells, where they are combined with proteins to create
chylomicrons |
Lacteals of villi |
Systemic circulation via lymph in thoracic duct |
| Short-chainfatty acids |
Simple diffusion |
Capillary blood in villi |
Liver via hepatic portal vein |
| Glycerol |
Simple diffusion |
Capillary blood in villi |
Liver via hepatic portal vein |
| Nucleic acids |
Active transport via membrane carriers |
Capillary blood in villi |
Liver via hepatic portal vein |
Electrolyte Absorption
The electrolytes absorbed by the small intestine are from both GI secretions and ingested
foods and liquids. Recall that electrolytes are compounds that separate into ions in
water. Most ions are absorbed throughout the entire small intestine. Exceptions are iron
and calcium, which are primarily absorbed in the duodenum.
In the small intestine, sodium ion absorption is linked via co-transport mechanisms to
the absorption of glucose and amino acids. A sodium-potassium pump moves sodium ions
from the basolateral side of small intestine epithelial cells into the bloodstream they
enter. Chloride ions are also absorbed via active transport. Changing osmotic gradients
passively move potassium ions across the intestinal mucosa via facilitated diffusion (or
via leaky tight junctions).
In general, all nutrients that enter the intestine are absorbed, whether we need them or
not. Iron and calcium are exceptions; they are absorbed in amounts that fulfill the
needs of our body. The iron ion needed for the production of hemoglobin is absorbed into
mucosal cells via active transport. Once inside mucosal cells, ionic iron binds to the
protein ferritin, creating iron-ferritin complexes that act as intracellular storage
facilities for iron. When the body has enough iron, most of the stored iron is lost when
worn-out epithelial cells slough off. When the body needs iron because, for example, it
is lost during acute or chronic bleeding, there is increased uptake of iron from the
intestine and accelerated release of iron into the blood. Because women experience
significant iron loss during menstruation, they have around four times as many iron
transport proteins in their intestinal epithelial cells as men.
Blood levels of ionic calcium (Ca2+) determine the absorption of calcium. The active form
of vitamin D, under the control of parathyroid hormone, provides local regulation of
this process in the intestines. When blood levels of ionic calcium drop, parathyroid
hormone (PTH) secreted by the parathyroid glands stimulates the release of calcium ions
from bone matrix and increases the reabsorption of calcium by the kidneys. PTH also
provokes the activation of vitamin D by the kidneys, which triggers greater calcium ion
absorption in the small intestine.
Vitamin Absorption
Vitamins in our food are absorbed in the small intestine. Some of the vitamin K and B
vitamins created by enteric bacteria are absorbed in the large intestine. Fat-soluble
vitamins (A, D, E, and K) are absorbed along with dietary lipids in micelles via simple
diffusion. In fact, you need some fat in your diet in order to be able to absorb
fat-soluble vitamins. Some of the B vitamins are not actually found in our food but are
made by gut bacteria. Most water-soluble vitamins are absorbed by simple diffusion ,
including most B vitamins and vitamin C. An exception is vitamin B12, which is a very
large molecule. Intrinsic factor secreted in the stomach binds to vitamin B12, creating
a combination that can bind to mucosal receptors in the terminal ileum, launching its
active uptake by endocytosis.
Water Absorption
Each day, about nine liters (9.8 quarts) of fluid enter the small intestine; about 2.3
liters are ingested, and the rest is from GI secretions. About 95 percent of water is
absorbed in the small intestine. The absorption of water in the alimentary canal occurs
via osmosis. Around 300 to 400 mL of water is absorbed per hour. Water can move easily
in both directions across the intestinal mucosa. The active transport of solutes into
mucosal cells establishes a concentration gradient that results in water moving into the
cells via the process of osmosis. As water enters the cells, it concentrates the solutes
left in the lumen, enhancing the absorption of those molecules absorbed by passive
diffusion. This means that the absorption of water is essentially linked to the
absorption of solutes, which, in turn, determines the absorptive rate of those salts and
sugars that are absorbed via diffusion. The water that moves into the mucosal cells is
followed by these substances along their concentration gradients.
The large intestine is the terminal part of the gastrointestinal tract. The
primary digestive function of this organ is to finish absorption, produce some vitamins,
form feces, resorb water and eliminate feces from the body.
The large intestine runs from the cecum, where it attches to the ileum, to the anus. It
borders the small intestine on three sides. Despite its being around half as long as the
small intestine – 4.9 feet versus 10 feet (1.5 – 3 meters) – it is called the large intestine because it is more than twice the diameter of
the small intestine, 2.5 inches versus one inch (6 cm versus 2.5 cm). The large
intestine is tethered to the posterior abdominal wall by the mesocolon, a double layer
of peritoneal membrane.
The large intestine is subdivided into four main regions: the cecum, the colon, the
rectum, and the anus. The ileocecal valve, located at the opening between the ileum in
the small intestine and the large intestine, controls the flow of chyme from the small
to the large intestine.
Large Intestine Anatomical Structures
Like the small intestine, the mucosa of the large intestine has intestinal glands
that contain both absorptive and goblet cells. However, there are several
notable differences between the walls of the large and small intestines. For
example, other than the anal canal, the mucosa of the colon is simple columnar
epithelium. In addition, the wall of the large intestine has no circular folds,
no villi, and essentially no enzyme-secreting cells. This is because most
nutrients are already absorbed before chyme enters the large intestine. The
large intestinal wall does have thicker mucosa and deeper – and more abundant –
glands that contain a vast number of goblet cells. These goblet cells secrete
mucus that eases the movement of feces and protects the intestine from the
effects of the acids and gases produced by enteric bacteria.
 Anatomical structures of the large intestine. This work by Cenveo is
licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomical structures of the large intestine. This work by Cenveo is
licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The stratified squamous epithelial mucosa of the anal canal joins with the skin
around the anus. This mucosa varies considerably from that of the rest of the
colon to accommodate the increased abrasion in this region. The anal canal's
mucous membrane is organized in longitudinal folds called anal
columns that house a grid of veins. Depressions between the anal
columns, called anal sinuses, secrete mucus when they are crowded
by feces. This facilitates defecation. The pectinate line is a
horizontal, jagged band that runs alongside the inferior margins of the anal
sinuses. The mucosa superior to this line is fairly insensitive to pain, while
the area inferior to this line is very pain-sensitive. The difference in pain
response is due to the fact that the superior region is innervated by visceral
sensory fibers, and the inferior region is innervated by somatic sensory fibers.
There are two superficial venous plexuses in the anal canal – one with the anus
and the other with the anal columns. Inflammation and distension of these
(hemorrhoidal) veins causes hemorrhoids, an itchy condition caused by the
swelling of these vessels.
Three features are unique to the large intestine: teniae coli, haustra, and
epiploic appendages. The teniae coli are three bands of smooth
muscle that make up the longitudinal muscle layer of the muscularis externa of
the large intestine, except at its terminal end in the rectum. Tonic
contractions of the teniae coli bunch up the colon into a succession of pouches
called haustra, which are responsible for the wrinkled appearance
of the colon. Attached to the teniae coli are small, fat-filled sacs of visceral
peritoneum called epiploic appendages (omental appendices). These
fatty pouches of peritoneum found in the serosa from the transverse colon
through the sigmoid colon.
Although the rectum and anal canal have no teniae coli or haustra, they do have
well-developed layers of muscularis externa muscle that create the strong
contractions needed for defecation.
Large Intestine Gross Anatomy
The first part of the large intestine is the cecum, a small sac-like
region that is suspended inferior to the ileocecal valve. This cecum is about
2.4 inches long. The appendix or vermiform appendix
(vermiform = “worm-shaped”, and appendix = “appendage”) is a winding, coiled tube that attaches to the
cecum. This 2-7 cm (~3 inch) long appendix contains lymphoid tissue and plays an
important role in immunity. Nevertheless, its twisted anatomy provides a haven
for the accumulation and multiplication of enteric bacteria. The
mesoappendix, the mesentery of the appendix, tethers it to the
inferior part of the mesentery of the ileum.
 Gross anatomy of the large intestine. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Gross anatomy of the large intestine. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The end of the cecum joins with the colon, a long tube with several
distinct areas. The ascending colon runs up the right side of the
abdomen. At the inferior surface of the liver, it takes a right-angle turn,
forming the right colic (hepatic) flexure and becoming the
transverse colon. The transverse colon runs across to the left
side of the abdomen. It then bends sharply at a point immediately anterior to
the spleen, forming the left colic (splenic) flexure. As the
descending colon, it runs down the left side of the posterior
abdominal wall. After entering the pelvis inferiorly, it becomes the s-shaped
sigmoid colon, which extends medially to the midline. Most of the colon is between the peritoneal membrane and the body wall,
except for the intraperitoneal transverse and sigmoid colons, which are tethered
to the posterior abdominal wall by mesocolons.
The sigmoid colon joins the rectum in the pelvis, near the third sacral vertebra.
The rectum is the final 8 inches (20 cm) of the alimentary canal.
It extends anterior to the sacrum and coccyx. Even though rectum is
Latin for "straight," this structure has three lateral bends that create a trio
of internal transverse folds called the rectal valves. These valves
allow passing of gas (flatus) while preventing the simultaneous passage of
feces.
The last part of the large intestine is the anal canal, which is
located in the perineum, completely outside the abdominopelvic cavity. This 1
inch (3 cm) long structure opens to the exterior of the body at the anus. The
anal canal includes two sphincters. The involuntary internal anal
sphincter is made of smooth muscle; the voluntary external anal
sphincter is skeletal muscle. Except when defecating, these two
sphincters are usually closed.
There are many species of bacteria that populate the large intestines. They
digest food products for which we do not have enzymes (such as plant materials)
and we absorb some of the nutrient products. They can also produce some vitamins
like vitamin K and B vitamins. A byproduct of bacterial fermentation (digestion)
is gas (flatus).
Although the bacteria provide additional nutrients from the food we ingest, they
can also infect us. Because of the large and diverse bacterial population that
resides in the large intestine, the large intestine also contains plentiful
lymphatic tissue to protect us from potentially harmful effects of resident
bacteria.
Most bacteria that migrate to the cecum from the small intestine have already been killed
by lysozyme and other antibacterial molecules, HCl, and protein-digesting enzymes. Those
that are still living, along with bacteria that come into the alimentary canal through
the anus, are referred to as the large intestine's (enteric) bacterial
flora. The more than 700 species of bacterial flora in the colon participate in
chemical digestion and absorption. The large intestine is also home to a number of
viruses and protozoans, at least 20 of which are known pathogens.
Most enteric bacteria are nonpathogenic commensals (also called
symbiotic, wityh benefit to both organisms) that cause no harm as long as they stay in
the gut lumen. A refined system prevents these bacteria from crossing the mucosal
barrier. First, certain bacterial components activate the release of chemicals by the
mucosa's epithelial cells, which recruit immune cells, especially dendritic cells, into
the mucosa. Dendritic cells open the tight junctions between epithelial cells and extend
probes into the lumen to evaluate the microbial antigens. The dendritic cells then
travel to neighboring lymphoid follicles in the mucosa and hand over the antigens to T
cells. This triggers an IgA antibody-mediated response in the lumen that blocks the
commensals from infiltrating the mucosa and setting off a far greater, widespread
systematic reaction.
The major digestive role of the large intestine involves propulsion--pushing fecal matter
toward the anus and then out of the body. Chyme, which stays in the large intestine for
12 to 24 hours, contains few nutrients. Enteric bacteria are responsible for a small
amount of digestion. The bacterial flora create vitamins required for normal metabolism,
such as certain B vitamins and vitamin K. Most of the remaining water and some
electrolytes (especially sodium and chloride) are recycled.
| Structure |
Action |
Outcome |
| Lumen |
Bacterial degradation of chyme components |
Breaks down undigested proteins, carbohydrates, and amino acids into substances
that can be absorbed and detoxified by the liver or eliminated in feces;
synthesizes vitamin K and some B vitamins |
| Mucosa |
Mucus secretionAbsorption |
Lubricates colon; protects mucosaAbsorbs water, solidifies feces, and helps
maintain water balance in body; absorbed solutes include ions and certain
vitamins |
| Muscularis externa |
Haustral churningPeristalsisDefecation reflex |
Muscle contractions move contents between haustrumsContractions of circular and
longitudinal muscles propel contents along length of colon; Propels contents
into sigmoid colon and rectumContractions in sigmoid colon and rectum eliminate
feces |
Example
Ileostomy
It may surprise you that the large intestine can be completely removed without
significantly affecting digestive functioning. For example, if colon cancer necessitates
the removal of the large intestine, an ileostomy can be
performed, in which the terminal ileum is moved out to the abdominal wall. A sac is then
attached to the abdominal wall to collect eliminated food residues.
Mechanical Digestion
Mechanical digestion in the large intestine begins when chyme moves from the
ileum into the cecum, an activity regulated by the ileocecal valve. This
sphincter is usually partially closed, allowing slow movement of chyme into the
large intestine. Right after we eat, peristalsis in the ileum is escalated by
the gastroileal reflex, which forces whatever chyme is in the ileum into the
cecum. The activity of the hormone gastrin also relaxes the ileocecal valve.
When the cecum is distended with chyme, contractions of the ileocecal sphincter
strengthen. Once chyme enters the cecum, colon movements begin. As food residues
pass the ileocecal valve, they fill the cecum and gather in the ascending
colon.
The presence of food residue in the colon stimulates slow-moving haustral contractions (haustral churning). These sluggish
segmentations, primarily in the transverse and descending colons, occur about
every 30 minutes and last about one minute. When a haustra is distended with
chyme, its muscle contracts, pushing the residue into the next haustra. The
movements also mix the food residue, which helps the large intestine absorb
water.
The large intestine also has peristaltic movements, but they are slower than in
more proximal portions of the alimentary canal, at a rate of from three to 12
contractions per minute. The third type of movement in the large intestine is
called mass movements (mass peristalsis). These drawn-out,
slow-moving, but strong peristaltic waves start around the middle of the
transverse colon and quickly force the contents of the colon into the rectum.
Mass movements usually occur three or four times per day, either while we eat or
immediately afterward. Distension in the stomach and the breakdown products of
digestion in the small intestine provoke the gastrocolic or
duogenocolic reflex, which increases motility, including mass
movements, in the colon. Fiber in the diet both softens the stool and increases
the power of colonic contractions, optimizing the activities of the colon.
Chemical Digestion
The glands of the large intestine secrete only mucus; they do not secrete
digestive enzymes. So chemical digestion in the large intestine occurs only
through the activity of the bacteria in the lumen of the colon. Bacteria ferment
residual carbohydrates in the chyme and discharge the hydrogen sulfide, carbon
dioxide, and methane gases that help create flatus (gas)
in the colon(flatulence refers to excessive flatus).
Some of these gases, including dimethyl sulfide, have foul odors. Each day,
about 500 mL of flatus is produced in the colon. Much more is produced when we
eat some fiber-rich foods such as beans.
After chyme has been in the large intestine for three to 10 hours, water absorption
changes it into the solid or semisolid substance called feces
("stool"). Feces is composed of undigested food residues (dietary fiber),
unabsorbed digested substances, millions of bacteria, sloughed-off epithelial cells from
the GI mucosa, inorganic salts, mucus, fat and just enough water to let it pass smoothly
out of the body. Of every 500 mL (17 ounces) of food residue that enters the cecum each
day, about 150 mL (5 ounces) becomes feces.
Although the small intestine absorbs around 90 percent of the water that enters the GI
tract, the water absorbed in the large intestine is important in maintaining the body's
water balance. For every 0.5 to 1.0 liter (16 to 32 ounces) of water that goes into the
large intestine, only around 100 to 200 mL (3 to 7 ounces) escapes being absorbed by
osmosis.
The process of defecation begins when mass movements force feces from the sigmoid colon
into the rectum, stretching the rectal wall and provoking the defecation reflex, which eliminates feces from the rectum. This
parasympathetic reflex is mediated by the spinal cord. It contracts the sigmoid colon
and rectum, relaxes the internal anal sphincter, and initially contracts the external
anal sphincter. The presence of feces in the anal canal sends a signal to the brain,
which gives us the choice of voluntarily opening the external anal sphincter
(defecating) or keeping it temporarily closed. When we decide to delay defecation, it
takes a few seconds for the reflex contractions to stop and the rectal walls to relax.
The next mass movement will trigger another defecation reflex and another until we
defecate.
Feces is eliminated during defecation through contraction of the rectal muscles. We help
this process by a voluntary procedure called the Valsalva
maneuver, in which we increase intra-abdominal pressure by contracting our
diaphragm and abdominal wall muscles and closing our glottis. Along with learned control
of the external anal sphincter, these actions must be learned, which is why infants and
young children wear diapers.
Diet, health, exercise and stress determine the frequency of bowel movements. The number
of bowel movements varies greatly between individuals, ranging from two or three per day
to three or four per week.
Lying outside the oral mucosa are the three pairs of major salivary
glands, which secrete the majority of saliva into ducts that open into the
mouth. The parotid glands lie between the skin and the masseter
muscle near the ears. They secrete saliva into the mouth through the parotid duct, which is located near the second upper molar tooth. The submandibular glands, which are in the floor of the mouth, secrete
saliva into the mouth through the submandibular ducts near the
lower central incisor. The sublingual glands, which lie below the
tongue, use the lesser sublingual ducts to secrete saliva into the
oral cavity.
 Salivary gland locations. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Salivary gland locations. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Different salivary glands secrete unique formulations of saliva according to their
cellular makeup. For example, the parotid glands secrete a watery solution that contains
salivary amylase. The submandibular glands have cells similar to those of the parotid
glands, along with some mucous-secreting cells. So saliva secreted by the submandibular
glands also contains amylase but in a liquid thickened with mucus. The sublingual glands
contain mostly mucous cells, and they secrete the thickest saliva with the least amount
of salivary amylase.
Example
Homeostatic Imbalances of the Parotid Glands: Mumps
Infections of the nasal passages and pharynx can attack any salivary glands. The
parotid glands are the usual site of infection with the virus that causes mumps (Paramyxovirus). Mumps manifest with enlargement and
inflammation of the parotid glands, causing the characteristic swelling between the
ears and the jaw. Symptoms include fever and throat pain, which can be severe when
swallowing acidic substances such as orange juice.
In about one third of men who are past puberty, mumps also causes testicular
inflammation, typically affecting only one testis and thus rarely resulting in
sterility. The incidence of mumps has dropped considerably since 1967, when a
vaccine was introduced.
The autonomic nervous system regulates salivation (the secretion of
saliva). An average of 1 to 1.6 quarts (0.9-1.5 liters) of saliva is secreted each day.
In the absence of food, parasympathetic stimulation keeps saliva flowing continuously to
moisten mucous membranes and lubricate tongue and lip movements when we talk; swallowed
saliva moistens the esophagus. Most saliva is reabsorbed, preventing fluid loss. During
times of stress, sympathetic stimulation takes over, reducing serous salivation and
causing dry mouth. When you are dehydrated, salivation stops to conserve water, causing
the mouth dryness that helps stimulate feelings of thirst. Having a drink restores body
water homeostasis and moistens the mouth.
Salivation from the major salivary glands is stimulated by both the taste and sensation
of food. Food contains chemicals that stimulate taste bud receptors on the tongue, which
send impulses to the superior and inferior
salivatory nuclei in the brain stem. These two nuclei then send back
parasympathetic impulses through fibers in the facial and glossopharyngeal nerves to
stimulate salivation. Even after we swallow food, salivation is increased to cleanse the
mouth and to dilute and neutralize any irritating chemical remnants -- such as that hot
sauce in your burrito. Salivation can also be stimulated by simply thinking about,
seeing, or smelling food.
Chemical digestion in the small intestine relies on the activities of three accessory
digestive organs: the pancreas, liver and gall bladder. The pancreas produces pancreatic
juice, which contains digestive enzymes and bicarbonate, and delivers it to the
duodenum.
The digestive role of the liver is to produce bile and export it to the duodenum. The
gall bladder primarily stores and releases bile.
The soft, tadpole-shaped pancreas is a gland that is about 5-6
inches (13-15 cm) long and 1 inch (2.5 cm) thick. It lies posterior to the greater
curvature of the stomach. The pancreas regions are described as the head, body, and
tail. The head is next to the duodenum. The body lies behind the stomach. The tail is in
contact with the spleen.
 The pancreas and common bile duct. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
The pancreas and common bile duct. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Pancreas Ducts
The pancreas is important in digestion, because it produces pancreatic juice, a combination of fluid and digestive enzymes.
Exocrine cells release this juice into small ducts that eventually unite to
create two larger ducts that deliver pancreatic juice to the small intestine.
The larger of these two ducts is the centrally located main
pancreatic duct (duct of Wirsung). In most individuals, this duct fuses
with the common bile duct from the liver and gall bladder before entering the
duodenum via the hepatopancreatic ampulla (ampulla of
Vater), a dilated common duct. This ampulla opens on the major duodenal
papilla, an elevation of the duodenal mucosa, which is situated approximately 4
inches (10 cm) inferior to the pyloric sphincter of the stomach. The smooth
muscle hepatopancreatic sphincter controls the flow of pancreatic juice and bile
into the small intestine. The second large pancreatic duct, the accessory duct (duct of Santorini) runs from the pancreas directly
into the duodenum, approximately 1 inch (2.5 cm) above the hepatopancreatic
ampulla.
Pancreatic Islets
In the pancreas, small clusters of glandular epithelial (secretory) cells
surround the ducts. The exocrine structures of the pancreas contains 99 percent
of the clusters that are called acini (singular - acinus).
Cells of the acini secrete pancreatic juice. The endocrine part of the pancreas
is made up of the remaining 1 percent of clusters, which are called pancreatic islets (islets of Langerhans). These cells
produce the hormones pancreatic polypeptide, insulin, glucagon, and
somatostatin. Insulin and glucagon are important in carbohydrate metabolism.
Panreatic Acini and Exocrine Digestive Function
Regulation of pancreatic secretion is the job of both hormones and the
parasympathetic nervous system. The hormone secretin, whose secretion is
stimulated by the presence of HCl in the intestine, provokes cells of the
pancreatic duct to release bicarbonate-rich pancreatic juice. The presence of
fats (and some proteins) in the intestine stimulates the secretion of the
hormone cholecystokinin (CCK), which then stimulates the release enzyme-rich
pancreatic juice and enhances the activity of secretin. As a result, much more
bicarbonate-rich juice is released in the presence of both hormones.
Parasympathetic regulation occurs mainly during the cephalic and gastric phases
of gastric secretion, when vagal stimulation prompts the secretion of pancreatic
juice.
The liver is the largest gland in the body, as well as one of the
most important organs. It plays a number of major roles in metabolism and regulation. In
an adult, the reddish-brown liver weighs about 3 pounds (1.4 kg) This wedge-shaped organ
lies inferior to the diaphragm in the right upper quadrant of the abdominal cavity. The
liver is almost completely surrounded by the rib cage, which offers it some
protection.
The Liver’s Anatomical Structures
The liver has different levels of structure and function, and different models
have been used to describe the organizational and functional relationships
between hepatocytes, bile canaliculi, and hepatic sinusoids. The hepatic lobule was considered the functional unit of the
liver in years past. In this model, each hexagonal (six-sided) hepatic lobule
has a central vein. Radiating from this vein are rows of hepatocytes and hepatic
sinusoids.
The hepatic acinus has become the preferred model of the
metabolic functional unit of the liver in recent years. Each primarily oval
hepatic acinus includes parts of two adjacent hepatic lobules. Branches of the
portal triad that run along the hepatic lobule border delineate the short axis
of the hepatic acinus. The long axis is created by two imaginary curved lines
that connect the two central veins nearest the short axis. In the hepatic
acinus, hepatocytes are organized in three zones around the short axis. Zone 1
cells, which are nearest the portal triad branches, are the first to receive
oxygen, nutrients, and toxic substances from incoming blood. After a meal, zone
1 cells are the first to absorb glucose and store it as glycogen; during
fasting, they are the first to break down glycogen to glucose. If circulation is
disrupted, zone 1 cells are the last to die and the first to regenerate. Zone 3
cells, which are farthest away from the portal triad branches, die first if
circulation is disrupted and are the last to regenerate. They are also the first
to demonstrate the accumulation of fat. Predictably, the structural and
functional qualities of zone 2 cells lie between those of zone 1 and 3
cells.
The popularity of the hepatic acinus model is based on its reasonable description
of glycogen storage and release patterns, as well as the degeneration,
regeneration, and toxic effects in the three zones according to their proximity
to portal triad branches.
Liver Gross Anatomy
The liver is divided into two primary lobes, a large right lobe and a smaller
left lobe. Separating the right and left lobes anteriorly is a mesentery called
the falciform ligament, which also helps suspend the liver in the abdominal
cavity. A number of anatomists believe that the right lobe also includes an
inferior quadrate lobe and a posterior caudate lobe, which are defined by
internal features. Ligaments connect the liver to other visceral organs, and the
visceral peritoneum enshrouds almost the entire liver, except for the part that
abuts the diaphragm. The lesser omentum tethers the liver to the lesser
curvature of the stomach. The porta hepatis ("gateway to
the liver") is where the hepatic artery and hepatic portal vein enter the liver on its inferior
surface. These two vessels, along with the common hepatic duct, run through the
lesser omentum on the way to their destinations.
 Gross anatomy of the liver. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Gross anatomy of the liver. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
The hepatic artery delivers oxygenated blood from the heart to the liver. The
portal system in general has a direct cconnection between two organs bypassing
the systemic circulation. The hepatic portal vein delivers deoxygenated blood
containing newly absorbed nutrients, drugs, and possible bacteria from the
alimentary canal. Branches of both vessels bring blood into liver sinusoids,
where hepatocytes take up most nutrients and some of the toxins. The hepatocytes
secrete substances, including nutrients needed by other cells, back into the
blood, which drains into the central vein and then the hepatic vein. In this
hepatic portal circulation, blood from the GI tract passes through the liver
before entering the systemic circulation. This is why the liver is a common site
for the metastasis of cancer that originates in the alimentary canal.
Liver Ducts
The liver has three main components: hepatocytes, bile canaliculi, and hepatic sinusoids.
Between adjacent hepatocytes, grooves in the cell membranes provide room for bile canaliculi ("small canals"). These small ducts accumulate the
bile produced by hepatocytes. From here, bile flows first into bile ductules and then
into bile ducts. The bile ducts unite and form the larger right
and left hepatic ducts, which themselves merge and exit the
liver as the common hepatic duct. This duct then joins with the
cystic duct from the gall bladder, forming the common bile duct
through which bile flows into the small intestine. Bile production increases when
fat-rich chyme stimulates the release of secretin from intestinal cells. Between meals,
when most absorption is complete, the hepatopancreatic sphincter prevents bile flow into
the duodenum, and bile backs up into the gall bladder, where it is stored until
needed.
Liver Circulation
Hepatic sinusoids are specialized blood vessels, more like dilated
channels, located between rows of hepatocytes. Branches of the hepatic artery deliver
oxygenated blood to hepatic sinusoids; while branches of the hepatic portal vein deliver
nutrient-laden blood. The hepatic sinusoids combine and send blood into a central vein. Blood then flows into the hepatic
veins, which drain into the inferior vena cava. This means that blood and bile
flow in opposite directions. The hepatic sinusoids also contain the immune cells called
Kupffer cells. A distinctive arrangement in the liver called
the portal triad includes three basic structures: a bile duct, a
hepatic artery branch, and a hepatic portal vein branch.
Liver Ligaments
The liver is covered with a visceral peritoneum. These ligaments are useful anatomic
positions but serve little physiological purpose aside from attaching the liver to the
body wall and other structures. The ligamentum teres (round ligament)
runs along the inferior border of the falciform ligament.
Tapered extensions of the parietal peritoneum, called the right and left coronary ligaments, hang the liver from the diaphragm.
The gall bladder stores and concentrates the bile produced by the
liver. The green colored gall bladder lies in a shallow area on the posterior surface of
the right lobe of the liver. This pear-shaped sac, which is about 3-4 inches (7-10 cm)
long, usually suspends from the anterior inferior margin of the liver. The gall bladder
is divided into three regions. The wide fundus runs inferiorly,
past the inferior border of the liver. The central portion of the gall bladder is called
the body, and the tapered portion the neck.
These two areas project superiorly.
 Gall Bladder. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
Gall Bladder. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United
States (http://creativecommons.org/licenses/by/3.0/us/).
The simple columnar epithelium of the gall bladder mucosa is organized in rugae, similar
to those of the stomach. There is no submucosa in the gall bladder wall. The wall's
middle, muscular coat is made of smooth muscle fibers. When these fibers contract, the
gall bladder's contents are released into the cystic duct.
Visceral peritoneum forms the outer coat of the gall bladder.
Movement of Bile
The ducts and mechanisms involved in the movement of bile.
The primary digestive role of the liver is to produce bile and deliver it to the
duodenum. Bile salts separate large fat globules that enter the small intestine into
millions of small fatty droplets with large surface areas that are amenable to the
activity of fat-digesting enzymes.
The pancreas produces pancreatic juice, whose high alkalinity helps neutralize acidic
chyme in the duodenum, while providing the ideal environment for the actions of
intestinal and pancreatic enzymes.
The digestive function of the gall bladder is to store and concentrate the bile produced
by the liver and then release it when it is needed in the small intestine. The gall
bladder's mucosa absorbs water and ions from bile, concentrating it by as much as
twenty-fold.
| Organ |
Function in the Digestive Process |
Outcome |
| Liver |
Produces bile, which includes bile salts |
Bile salts emulsify lipids for and digestion and absorption |
| Pancreas |
Produces pancreatic juice and sends it to the duodenum via the pancreatic
duct |
Pancreatic juice contains a variety of digestive enzymes |
| Gall bladder |
Stores and concentrates bile |
Sends bile to the duodenum via the common bile duct |
| Salivary Glands |
Produce saliva |
Begin to break down charbohydrates and to cleanse the mouth |
With the exception of oxygen from the respiratory system and sunlight (for vitamin D
formation) from the integumentary system, we obtain all of the materials we need for
growth and energy from the digestive system. It is through the digestive system that we
obtain water, nutrients and other essential building blocks for metabolism. However, as
you know from day to day eating habits, the input into the digestive system can change
greatly in amount, frequency and content. So it is up to the digestive system to have
complex processes in place that help maintain homeostasis at various levels in the
body.
Hormonal control (for long distance regulation) and paracrine control (for short
distance, or local, regulation) help maintain processes throughout the digestive
system that regulate secretion of chemicals, mechanical changes and absorption
of nutrients.
| Hormone/Paracrine |
Production Site |
Production Stimulus |
Target Organ |
Action |
| Cholecystokinin (CCK) |
Duodenal mucosa |
Fatty chyme, partially digested proteins |
Liver, pancreas, gall bladder, Hepato-pancreatic sphincter |
Potentiates activity of secretin; increases production of enzyme-rich
pancreatic juice; contracts gall bladder and releases bile; relaxes
sphincter, permitting bile and pancreatic juice to enter the
duodenum |
| Gastrin |
Stomach mucosa |
Presence of food in stomach; release of acetylcholine by axons |
Stomach, small intestine, ileocecal valve, large intestine |
Increased secretion by gastric glands; provokes gastric emptying; provokes
intestinal muscle contraction; relaxes valve; provokes mass movements |
| Histamine |
Stomach mucosa |
Presence of food in stomach |
Stomach |
Stimulates parietal cells to release HCl |
| Intestinal gastrin |
Duodenal mucosa |
Presence of acidic and partially digested foods in duodenum |
Stomach |
Activates gastric glands; stimulates motility |
| Motilin |
Duodenal mucosa |
Fasting; neural stimuli activate release every 1.5 to 2 hours |
Proximal duodenum |
Activates migrating motility complex |
| Secretin |
Duodenal mucosa |
Primarily acidic chyme |
Stomach, pancreas, liver |
Restricts gastric gland secretion and gastric motility in gastric phase
of secretion; increases production of pancreatic juice rich in bicarbonate
ions; enhances activity of CCKEnhances bile production |
| Serotonin |
Stomach mucosa |
Presence of food in stomach |
Stomach |
Contracts stomach muscle |
| Somatostatin |
Mucosa of stomach and duodenum, pancreas |
Presence of food in stomach; sympathetic axon stimulation |
Stomach, pancreas, small intestine |
Restricts all gastric secretions; restricts gastric motility and
emptying; restricts secretion; inhibits intestinal absorption by restricting
GI blood flow |
| Vasoactive intestinal peptide |
Duodenal mucosa |
Chyme with partially digested foods |
Duodenum, stomach, small intestine |
Activates buffer secretion; dilates intestinal capillaries; restricts HCl
production; relaxes intestinal smooth muscle |
Neural innervation of the GI tract is provided intrinsically by the enteric nervous
system and extrinsically by the autonomic nervous system. The enteric
nervous system contains around 100 million motor neurons, sensory neurons, and
interneurons that run from the esophagus to the anus. These neurons are grouped into two
plexuses. The myenteric plexus (plexus of
Auerbach) lies in the muscularis externa layer of the intestinal wall while the
submucosal plexus (plexus of Meissner)
lies in the submucosal layer. The myenteric plexus is responsible for GI tract motility (spontaneous movement), especially the rhythm and force
of contractions of the muscularis. The submucosal plexus regulates digestive secretions
and reacts to the presence of food.
The autonomic nervous system provides parasympathetic and sympathetic innervation to the
GI tract. In general, parasympathetic nerves increase GI secretion and motility by
stimulating neurons of the enteric nervous system, and sympathetic nerves decrease GI
secretion and motility by restricting the activity of enteric nervous system neurons.
When we are angry, frightened, or anxious, sympathetic innervation of the GI tract is
stimulated, slowing digestive activity. Many people feel ‘butterflys’ in their stomach
(or regurgitate) when they are nervous.
Many enteric nervous system neurons are part of the GI reflex pathways
responsible for controlling secretion and motility in response to GI tract stimuli.
In these pathways, enteric nervous system sensory neuronal axons synapse with neurons of
the enteric, central, or autonomic nervous system, either activating or inhibiting the
activities of GI glands and smooth muscle.
Example
Homeostasis of Water
As an example of homeostasis maintained in the digestive system, we
will consider the homeostasis of water. In the introductory units, we
covered all
of the important functions that water plays in our bodies. We get
water from drinking
liquids and from most of the foods that we eat. Water is necessary
for normal
functioning of most of our tissue systems including cardiovascular
fluids (blood). We
lose water by exhalation (respiratory system), urination (urinary
system) and through
feces in the digestive system. A fraction of water is retained in the
digestive system
to maintain movement, and water is both absorbed and secreted in the
large intestine
[link to section above on water absorption.
Infections of the gastrointestinal tract leading to diarrhea:
Diarrhea is a gastrointestinal disorder characterized by frequent watery stools.
Diarrhea can occur secondary to a number of problems, including bacterial or viral
infection of the gastrointestinal tract. The most common causes of these infectious
diarrheas are infections with the Escherichia coli or Vibrio cholera bacteria or from
Rotavirus.. In the large intestine, 98% of digestive system water is reabsorbed along
with electrolytes and nutrients. Bacterial or viral infectioninterferes with the
processes that control fluid reabsorption, leading to frequent, watery stools.
Infectious diarrheal disease usually occurs in older infants and children, particularly
in situations where safe sources of drinking water are not available. Most children can
recover from these gastrointestinal infections; however, diarrhea can lead to loss of
substantial body fluids, sodium, chloride and other electrolytes. The loss of just 10%
of body fluids can prevent maintenance of blood pressure. Diarrhea leads to 2.2 million
deaths per year throughout the world, most in children in developing countries.
Cholera is a severe diarrheal disease caused by the Vibrio cholerae
bacterium. This bacterium produces a toxin that binds tightly to cells of the large
intestine and prevents water absorption. Simply providing water to cholera victims does
not prevent dehydration, but adds to the volume of diarrhea. Proper treatment solutions
contain water, salts and glucose since all of these are responsible for absorption of
fluids.
In addition to serving as a nutritive input for all of the body
systems, the digestive system interacts with all of the systems of the
body.
| Body System |
Digestive System Support |
Digestive System Benefits/Effects |
| Cardiovascular |
Provides nutrients for the formation of plasma protein and
blood cells; the liver detoxifies blood, produces plasma proteins, and
destroys old red blood cells; Hydration levels to help maintain blood
pressure |
Blood vessels transport nutrients from the digestive system to other parts of body; blood supplies digestive organs |
| Endocrine |
Stomach and small intestine produce hormones |
Endocrine hormones help regulate secretion in digestive glands
and accessory organs; insulin and glucagon control glucose storage in
liver |
| Lymphatic |
Provides nutrients for lymphoid organs; stomach acidity blocks entry of pathogens |
Lacteals absorb fat; Peyer's patches block entry of pathogens |
| Muscular |
Provides glucose for muscle action; liver metabolizes lactic acid buildup after muscle activity |
Smooth muscle contraction creates peristalsis; skeletal
muscles support and protect abdominal organs; Skeletal muscles aid in
swallowing and voluntary sphincter control |
| Nervous |
Provides nutrients for neurons |
Nerves innervate smooth muscles responsible for digestive tract movements |
| Reproductive |
Provides nutrients for reproductive organs and fetal development |
During pregnancy, digestive organs are crowded, causing heartburn and constipation |
| Respiratory |
Shared pharynx allows breathing through the mouth |
In lungs, gas exchange provides oxygen to, and excretes carbon dioxide from, the digestive tract via the vascular system. |
| Skeletal |
Provides calcium and other nutrients for bone growth and repair |
Bones protect and support digestive organs; hyoid bone helps deglutition |
| Integumentary |
Provides nutrients required by the skin, hair and nails |
The skin helps protect digestive organs and provides vitamin D for calcium absorption |
| Urinary |
Liver synthesizes urea; digestive system excretes bile pigments from liver |
Kidneys convert vitamin D to its active form, allowing calcium
absorption; Urninary water loss influences water available for saliva
formation and chime production |
All metabolic processes in the body require nutrients and oxygen and
produce waste products. Nutrients are absorbed through the digestive
system and oxygen is acquired through the respiratory system. The
cardiovascular system is responsible for moving material through the
body. The heart pumps blood throughout the blood vessels.
Common Dysfunctions of the Cardiovascular System
In this unit, we will discuss many different cardiovascular
disorders.
Blood Disorders
The variety of disorders that affects blood highlights how physiology can
be impacted by genetics, environmental changes, pathogens or a
combination of all of these factors. For example, digestive diseases or
prolonged poor nutrition can lead to a loss of blood protein called
hypoalbuminemia. There are numerous genetic conditions in blood which
cause coagulation (blood clots) inside the body, which can be exacerbated
by poor diet and lack of exercise. Also, there are numerous genetic
anemias (insufficient number of red blood cells) including thalassemia
that are found in regions of the word prone to malaria infection. Malaria
is an example of a pathogenic infection of blood cells.
Vascular Disorders
Vascular disorders including hypertension (high blood pressure) are
primarily a function of lifestyle including diet, exercise and stress.
Recent studies have shown that genetics also plays a role in the presence
and progression of these disorders.
Disorders of the Heart
Your heart will beat billions of times in your life, on average once per
second; an electrical impulse will cause a mechanical contraction. If the
heart conduction system or mechanical system falters, it can lead to
devastating changes in heart physiology and whole body physiology. Even
if the heart weakens over time it can have damaging effects since the
heart never gets a chance to rest.
The heart pumps continuously to provide tissues and organs with needed oxygen and remove
cellular waste materials. On average, the heart pumps 70 to 72 beats per minute, every
minute of every day. It is able to pump close to the entire volume of blood in your body
every minute. This means that on a daily basis, almost 7200 liters of blood are pumped by
the heart. This amount increases when you exercise.
The heart is separated from the lungs inside the thorax by a membranous sack called the
parietal pericardium. This sack is continuous with the outermost layer of
the heart called the visceral epicardium. Just deep to the pericardium is the
myocardium, which is the muscle layer of the heart. It comprises 99% of the
mass of the heart. The endocardium is the innermost layer of the heart tissue
that lines the heart chambers and is continuous with the endothelium lining blood
vessels.
Heart Conduction
The heart’s ability to generate its own beat is an intrinsic quality. Inside the
superior wall of the right atrium a group of specialized cells called the sinoatrial
(SA) node spontaneously depolarize, creating a signal that passes to the atria and
the ventricles, causing their contraction. Because the SA node repeatedly creates
this signal, it is often called the pacemaker of the heart. These action potentials
create the rhythmic contraction of the heart that pushes blood through the heart and
circulatory system.
If the SA node becomes dysfunctional, the atrioventricular (AV) node,
found in the septal wall between the atria and ventricles can take over the pacing of
the heart. Its cells also exhibit the property of spontaneous depolarization, but it
does so at a slower rate than the SA node; thus the SA node typically “wins” the
pacing race by generating action potentials at a higher rate. This is called
overdrive suppression.
Because of this intrinsic quality, the heart is said to have
automaticity, meaning it is able to self-generate a beat. The heart
also has rhythmicity, which means it can generate its own pace of
beating. In fact, if a healthy heart is removed from a person or animal, it will
continue to beat outside of the body as long as it is put in a medium that supplies
it with energy. This occurs as hearts are transported for transplantation.
Figure 1
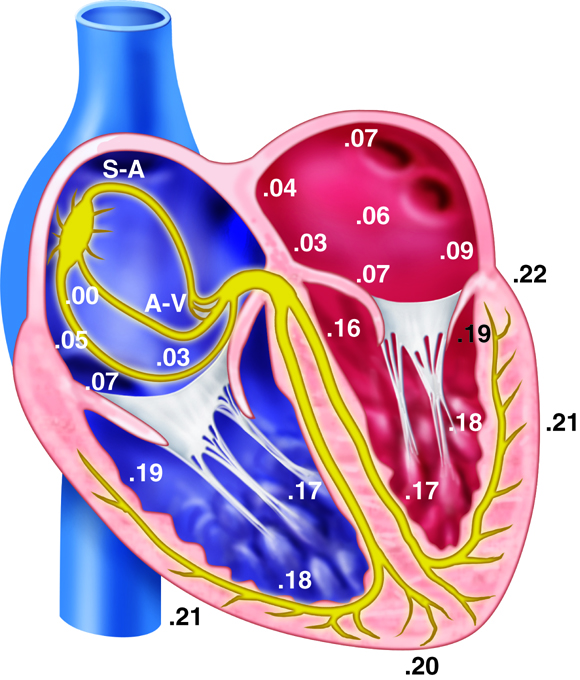
Action Potentials and Spontaneous Depolarization
Although the heart can intrinsically generate its own rate of spontaneous
depolarization (and therefore heart rate), this rate is under significant influence
by the autonomic nervous system. The sympathetic and parasympathetic branches of the
autonomic nervous system innervate the SA and AV nodes, adjusting their rates of
depolarization. The sympathetic nervous system will increase SA node depolarization
rates, increasing heart rate. The parasympathetic nervous system has the opposite
effect. At rest, the parasympathetic nervous system exhibits greater control of heart
rate. If this parasympathetic influence is removed, the heart rate typically
increases to about 100 beats per minute. Similarly, the pacemaker cells within the AV
node also spontaneously depolarize, but they do so at a rate of about 50 beats per
minute.
Electrical Conductance of the Heart
When the cells in the SA node depolarize, the action potential that is initiated is
spread throughout the heart. The signal travels through the heart muscle in two ways:
(1) from node to node along the conducting cells through the internodal
conducting pathway and (2) directly by cell–to-cell spread. The internodal
conducting pathway is actually made up of 3 separate paths of conducting cells that
connect the SA node with the AV node. Once the signal reaches the AV node, it passes
through the septum by conducting through the Bundle of His and into the Purkinje
Fibers. From here the signals are distributed throughout the ventricular myocardium
in rapid fashion.
Not only do action potentials travel through the internodal conducting cells from
node to node, but they also travel directly from cell to cell in the atria and
ventricles themselves. Therefore, when cells of the SA node depolarize, the cells
surrounding the SA node also depolarize. Because of the gap junctions between the
cells, the electrical signal travels quickly throughout all the individual muscle
cells of the atria.
The electrocardiogram, also called an ECG or EKG, measures the electrical
activity associated with the heart. By placing a minimum of three electrodes on the skin in
three locations—for example, the left arm, the right arm, and left leg—the electrical
signals that initiate contraction of the atria and ventricles can be recorded. These
recordings, or waveforms, are seen on a monitor and are called a tracing. The
resultant waveform consists of P, QRS, and T waves. These deflection waves are
indicative of the depolarization electrical activity just before the contraction of the
atria (P wave), the depolarization electrical activity stimulating ventricular contraction
(QRS complex), and repolarization of ventricles prior to relaxation (T wave).
Figure 2
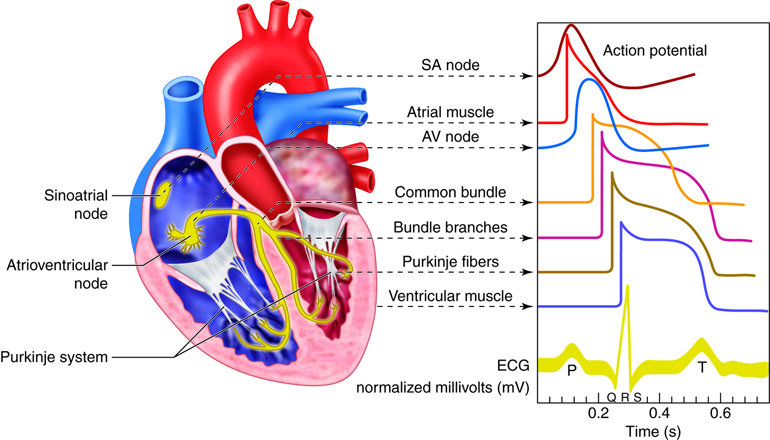
ECG tracings can provide information about the anatomy and placement of the heart in the
chest cavity. It provides information about the size of the chambers and the rhythm of
contraction. Importantly, ECGs are used to detect changes that have occurred in heart
function. Example changes include changes in the rhythm, changes in the conduction pathway
of electrical signals, or the development of ischemic areas (areas lacking oxygen) that
result from narrowed or occluded coronary arteries.
Changes in the tracing can be used to diagnose many diseases. The P-R interval measures the
time from atrial to ventricular contraction. Alterations to the length of this interval can
be indicative of damage in AV conductance that is caused by inflammation or medications.
The QRS wave shows the electrical activity that initiates ventricular contraction or
ventricular systole. Lengthening of this interval can indicate problems with ventricular
electrical conduction. During the S-T interval, the ventricles are depolarized and
contracting. Normally, the EKG reads zero voltage during the S-T interval, and a value
higher or lower than this can indicate that underlying ischemic damage to the heart may
have occurred. Ischemic damage results when there is a lack of sufficient oxygen to the
heart. The duration of the S-T interval varies with heart rate, decreasing at higher
rates.
Figure 3
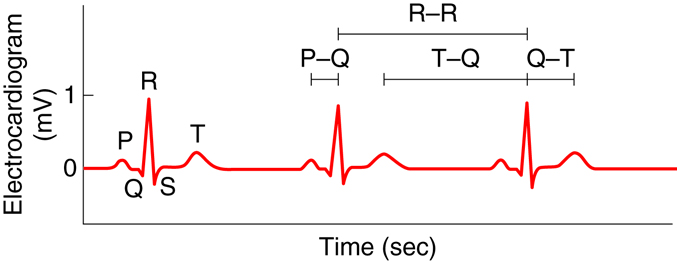
Component Amplitute (mV) Duration (sec)
| Component |
Amplitude (mV) |
Duration (sec) |
| P wave |
0.2 |
0.10 |
| QRS complex |
1.0 |
0.08-0.12 |
| T wave |
0.2-0.3 |
0.16-0.27 |
| P-Q interval |
N/A |
0.12-0.21 |
| Q-T interval |
N/A |
0.30-0.43 |
| T-Q segment |
N/A |
0.55-0.70 |
| R-R interval |
N/A |
0.85-1.00 |
If the SA node fires rapidly, the person has tachycardia. In one form of tachycardia,
atrial tachycardia, the atrial rate of contraction might actually be much higher than the
ventricular rate of contraction. This can happen because the AV node can limit the number
of action potentials that pass from the atria to the ventricles. On an ECG this would be
detected as more P waves than QRS complexes. If the ventricles are continuously firing
without a consistently preceding P wave, then the person would have ventricular
tachycardia. In this case the QRS complexes also look abnormal due to the pathway
of conduction having changed. These are just a few examples of the many abnormalities that
can be diagnosed using ECG tracings.
Diseases of the Heart: Arrhythmias
Arrhythmias (or dysrhythmias) are the abnormal rhythms in heart rate or
conduction pathway. Some tend to be harmless while others can be fatal. Arrhythmias
can range from having an occasional extra beat or a skipped beat to having
fibrillation of the atria or ventricles. Arrhythmias can also arise when the heart
either beats too slowly (bradycardia) or too quickly (tachycardia).
Arrhythmias are much more serious and deadly when the ventricle beats in an
uncontrolled manner. This arrhythmia, called ventricular fibrillation, does not
produce a productive heart contraction and will be fatal if not corrected. Atrial
fibrillation, on the other hand, is a common arrhythmia arising when multiple cells
outside of the SA node spontaneously depolarize at high rates. In this case the atria
quiver instead of beat, but because most of the blood in atria move into the
ventricles passively due to pressure differences even before the atria contract, it
is not nearly as damaging as ventricular fibrillation. People at risk of atrial
fibrillation still need to be managed because the condition increases their risk of
thromboemboli (blood clots that move to other parts of the body) and stroke. Both
atrial and ventricular fibrillation can be diagnosed on an ECG tracing as they will
present with either a complete lack of P waves or QRS complexes, respectively.
The cardiac cycle can be divided into two distinct phases, diastole and
systole. During diastole, the muscles are relaxed and the chambers fill
passively with blood. Although there is both an atrial and a ventricular diastole, which
differ slightly in their timing, the word diastole is commonly used in reference to
ventricular diastole. Systole is when the heart muscles contract. Atrial
systole helps fill the ventricles while ventricular systole is responsible for pushing the
blood into the pulmonary artery and aorta.
Figure 4
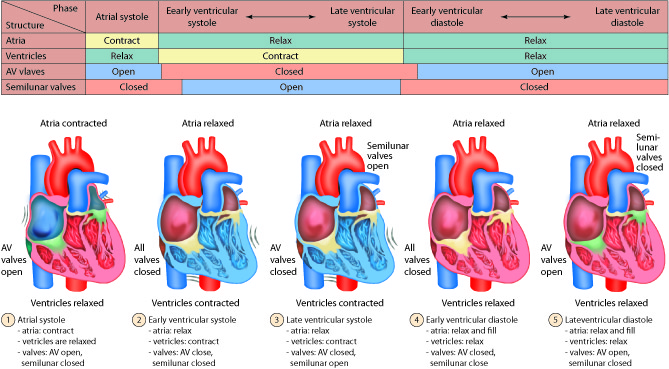
Diastole: Relaxation
During atrial diastole blood enters the left atrium from the pulmonary vein and the
right atrium from the inferior and superior vena cava. As ventricular diastole
begins, the atria are already well into their diastolic period. The ventricles have
just finished contracting, so the ventricular pressure is dropping as relaxation
begins. When the pressure within the ventricles drops below the atrial pressure, the
AV valves open. Blood passively pours into the atria and flows through the
open AV valves into the ventricles. As the blood collects in the ventricle, the
pressure builds. In order to continue filling the ventricle , the SA node
depolarizes, initiating atrial systole. The contraction of the atrium forces more
blood into the ventricle in diastole. Nearly 90% of the blood passively flows into
the ventricles while the remaining 10% enter due to atrial systole.
Systole: Contraction
The increasing volume of blood in the ventricles increases the pressure in the
ventricles. When this pressure exceeds that in the atria, the AV valves are forced
closed. The closing of these AV valves creates the first heart sound
(lub). By this time, the atria have finished systole and have entered their diastolic
period. There is a delay of about 0.1 sec (100 milliseconds) between the contraction
of the atria and the contraction of the ventricles. This delay provides enough time
for the atria to complete their contraction before the ventricles begin their turn
and prevents the chambers from competing with each other. Imagine if the atria and
ventricles contracted all at once. The blood flow would not be as controlled or
efficient.
The heart contracts in a repeatable sequence. First the atria contracts (as described
above), then the ventricles. This, along with the heart valves, allows the blood to
flow in one direction. When the ventricles begin to contract at the onset of systole,
both the AV and semilunar valves on either side of the ventricles are closed. This is
called isovolumetric contraction. The volume of blood in the ventricles
does not change because all the valves are closed, so there is no place for the blood
to go. As a result, isovolumetric contraction increases the pressure in the
ventricles. When the ventricular pressures exceed the arterial pressures (pulmonary
and aortic), the pulmonary and aortic semilunar valves open, ejecting blood into the
respective blood vessels. Once the ventricles enter diastole, the ventricular
pressures fall. When these pressures fall below that in their respective arteries,
the valves close again. This creates the second heart sound (dub).
Systole is now over and another diastole has begun; the cycle continues again.
Figure 5
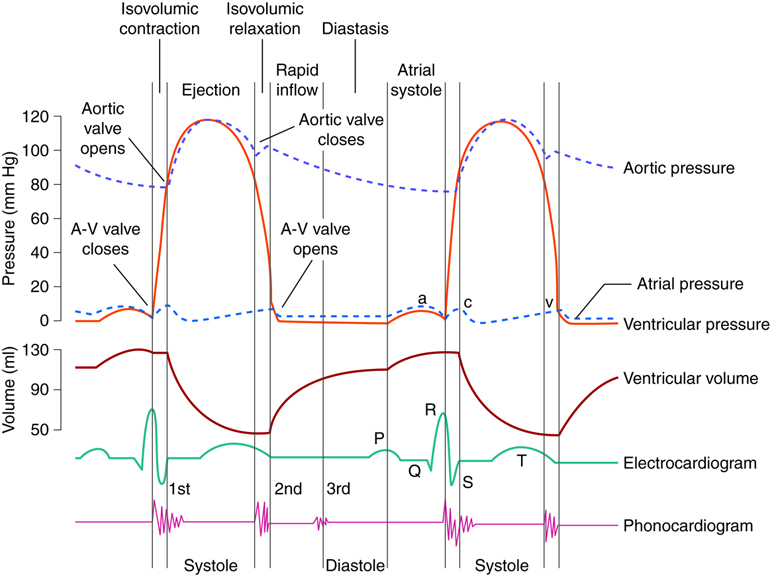
Cardiodynamics and Cardiac Output
Cardiac output is one measure of the heart's efficiency. It is the amount of blood
pumped per minute. Heart rate fluctuations are one way for the body to adjust the
cardiac output so that it can appropriately meet the demands of the body. When
resting, the body doesn’t have as high of a demand for oxygen because the muscles and
organs are at a lowered metabolic rate compared to when they are in a state of
exertion. Therefore, the heart rate is low. Similarly, if the body is actively
exercising, the demand for oxygen and the need to get rid of carbon dioxide are both
quite high. The heart rate, and therefore the cardiac output, increases
accordingly.
Control of Cardiac Output Through a Changing Stroke Volume
Cardiac output is the product of heart rate (beats per minute) and stroke volume
(volume of pumped blood per beat). Although the heart has the intrinsic ability to
generate a consistent heart rate, we have already learned that the autonomic nervous
system exerts significant control over this variable. Similarly, there are factors
that affect the stroke volume of the heart. The amount of blood that flows into the
heart is termed preload. Besides preload, the stroke volume of the heart
can also be affected by myocardial contractility, and, under certain conditions,
afterload, which is the pressure the ventricle needs to develop to eject blood.
We have already discussed heart rate. It is the pace the heart beats or the number of
beats per minute. It is easy to see how an increase or decrease in the number of
beats can affect the overall cardiac output. If the heart beats more often, more
blood will be pumped through the heart. If the cardiac output is a function of heart
rate times the stroke volume, then as the heart rate increases, so does the cardiac
output. At least this is true up to a point. At very high heart rates the heart may
not have time to fill appropriately during diastole, which can actually decrease
cardiac output. This is one of the risks of tachyarrhythmias (tachycardias).
Preload is the amount of blood in the heart immediately before it contracts (end
diastolic volume) and is mainly dependent on the flow rate of blood through the veins
(venous return). Venous return is increased when the systemic venous pressure
increases. For example, when we run the skeletal muscle in the legs contract,
pressing against the veins in the leg. This increases the pressure in the veins and
increases the flow of blood back to the heart. Another method to increase venous
pressure is through medications that stiffen (decrease compliance) of the veins.
Because veins can act as a storage space for blood, decreasing the amount of blood
that they are holding pushes more blood toward the heart, increasing preload. In both
of these cases (exercise or medications), stroke volume will increase, driving up
cardiac output. There is a molecular level explanation for this phenomena related to
the sarcomere structure in the muscle.
We know that in skeletal muscles, increased stretch in the muscle will increase the
force of the contraction, but only up to a certain point. Beyond that point, when the
number of actin-myosin cross bridges decrease, the force generated decreases.
A similar principle holds true with cardiac muscle. The muscle can be stretched by
adding more preload. Such stretching will increase the force of contraction by
providing more optimal overlap for crossbridge generation. The concept that the force
of heart muscle contraction will increase with an increase in end-diastolic
ventricular volume is the basis of the Frank-Starling Relationship. This
relationship was experimentally derived from the work of both O. Frank and E.H.
Starling (not by one person named Frank Starling). Together, they discovered that the
heart will adjust to an increase in volume by increasing its force of contraction. If
the volume decreases, the cardiac output will, likewise, decrease.
Figure 6
Even if preload doesn’t change, SV can either increase or decrease with a change in
contractility. Contractility differs from the Frank-Starling mechanism in that it
doesn’t affect the initial overlap of actin and myosin, like adding more preload
does. Instead contractility changes the force the myocardial cells generate by
adjusting the amount of calcium in the cytosol. An increase in contractility
increases the levels of cytosolic calcium, whereas a decrease in contractility has
the opposite effect. Because calcium is necessary to allow a crossbridge to form
(overlap isn’t enough), more cytosolic calcium maximizes the number of crossbridges
that have the potential to form. As a hypothetical example, let’s assume that a
myocardial cell has the ability to form 1000 crossbridges at its current preload.
Under normal conditions about 600 crossbridges will actually form because that is how
much cytosolic calcium is available during systole. If contractility increases, the
cell may now be able to form 800 crossbridges, increasing its force of contraction
and increasing its stroke volume.
Contractility is mainly affected by the sympathetic branch of the autonomic nervous
system. The greater the number of signals the myocardial cells are receiving from the
sympathetic nerves, the higher the contractility becomes. If the sympathetic nervous
system decreases the number of signals sent, contractility will decrease. Various
heart disorders will also decrease the heart’s contractility.
The last factor that influences the intrinsic regulation of the heart is the
afterload. The afterload is the pressure in the aorta that the heart has
to overcome to eject blood. Afterload is approximated by the mean arterial pressure.
For most individuals, afterload has little effect on stroke volume until mean
arterial pressure rises about 50% above normal. But for those with heart disease,
rising afterload at near normal pressures might decrease their stroke volumes.
The volume of blood that moves through any vessel or tissue in a given amount of time is
called blood flow. Blood flow is commonly measured in milliliters per minute
(mL/min) or liters per minute (L/min). The amount of blood that moves through either the
systemic or pulmonary vessels in one minute is the total blood flow or
cardiac output. Cardiac output is fairly constant when the body is at rest.
However, the needs of individual organs determine how much blood flows through that organ,
so if one or more organs needs more flow, cardiac output increases.
Blood Pressure
Blood pressure (BP) refers to the force per unit area that blood exerts
on a vessel wall. It is measured in millimeters of mercury (mmHg). Therefore, the
pressure from a column of mercury that is 120 mm high is equivalent to a BP of 120
mmHg. Clinically, such as when your BP is checked by your physician, it refers to
systemic arterial blood pressure in the biggest arteries close to the heart, where BP
is similar to that generated by the heart. From these large arteries, blood pressure
continuously decreases along each path that blood flow can take through the vascular
system. Once the blood gets back to the heart, it is at a very low pressure (near 0
mmHg). The heart then gives it a new “boost” of energy, raising BP again. It is this
pressure gradient that continues moving blood as blood moves from areas of higher
pressure to areas of lower pressure.
The highest BP is in the aorta and the bigger systemic arteries. In a healthy young
adult, BP increases to around 110 mmHg during ventricular systole (contraction) and
decreases to around 70 mmHg during ventricular diastole (relaxation). The highest
arterial pressure achieved during systole is called the systolic blood
pressure. The lowest arterial pressure reached during diastole is the
diastolic blood pressure. The pressure of blood steadily decreases as
blood travels farther and farther away from the left ventricle. In the blood that
moves from systemic arteries into systemic arterioles and then into capillaries,
pressure drops to approximately 35 mmHg. In capillaries, there are no BP
fluctuations, but pressure falls to around 16 mmHg when blood reaches the venous end
of capillaries. Because of their distance from the left ventricle, BP in systemic
venules and veins drops further. By the time blood reaches the right ventricle, its
pressure is typically 0 mmHg.
Figure 7
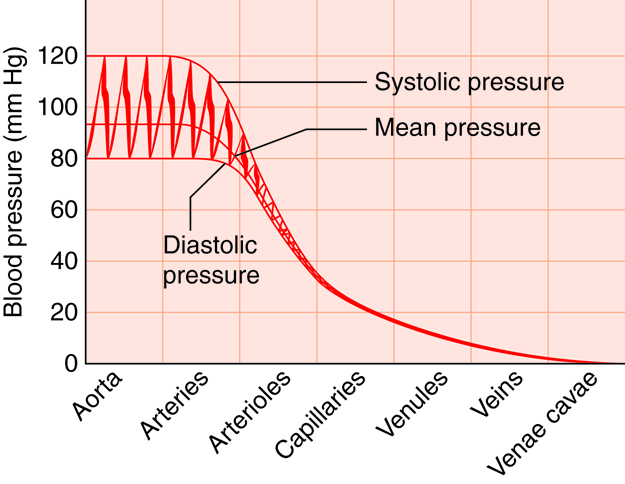
Pulse rate and blood pressure are good indicators of the functioning of the
circulatory system. After every systole of the left ventricle, arteries expand
and recoil. This creates a moving wave of pressure called the
pulse. The pulse rate matches the heart rate, which is
from 70 to 80 beats per minute when the body is at rest. Your pulse can be
checked in any artery that is close to the skin surface and that can be
compressed against a solid structure such as a bone. The pulse rate is commonly
checked at the site of the radial artery at the inner wrist or at the site of
the carotid artery at the neck. The pulse can also be strongly felt at the
femoral artery in the groin, at the brachial artery between the bicep and
tricep, and at the dorsalis pedis artery on the top of the foot. The strongest
pulse is in arteries near the heart. A rapid resting heart rate, indicated by a
rapid pulse rate, of over 100 beats per minute is called
tachycardia. A slow resting heart/pulse rate of less than 50
beats per minutes is called bradycardia. Bradycardia is normal in
endurance-trained athletes such as marathon runners.
Blood pressure is typically assessed in the brachial artery of the left arm. An
inflatable rubber cuff with attached squeezable bulb used to measure BP is
called a sphygmomanometer. The bulb is squeezed until compression
stops the flow of blood in the brachial artery. Then the cuff is slowly
deflated. When the artery reopens, blood spurts through the vein, making a
sound that corresponds to systolic blood pressure. With continuing deflation,
the sound of the flowing blood ultimately goes away, which corresponds with
diastolic blood pressure.
Figure 8
Blood Pressure Calculations
Average arterial BP, or mean arterial pressure (MAP), is about
one-third of the difference between the systolic and diastolic BP. MAP is
determined using the following equation:
So someone with a BP of 120/80 mmHg would have an MAP of approximately 98 mmHg (i.e., 80 + 1/3(120 − 80).
You have already learned that cardiac output can be calculated by multiplying
heart rate by stroke volume. Cardiac output can also be estimated by dividing
MAP by the resistance of the peripheral circulation (i.e. total peripheral
resistance (TPR), or systemic vascular resistance (SVR)) , using the following
formula:
The same equation can be reconfigured to estimate MAP:
This means that if stroke volume or heart rates increases, the subsequent
increase in cardiac output will result in an increase in MAP, assuming
resistance does not change. Similarly, reduced cardiac output results in a
reduced MAP, again assuming a constant amount of resistance.
The total volume of circulating blood also affects BP. You may recall the the
total amount of blood in the vascular system contributes to venous return. We
have learned through the Frank-Starling mechanism that as venous return
increases, so does stroke volume and therefore cardiac output. A healthy adult
has about 5 liters (5.3 quarts) of blood. If there is significant bleeding,
volume decreases. If the blood volume reduction is less than 10 percent,
homeostatic mechanisms kick in to help normalize BP. If blood volume drops by
more than 10 percent, the decrease in venous return causes BP to fall.
Similarly, conditions that increase blood volume (for example, water retention)
usually result in an increased BP – as long as the individual has a healthy
heart to pump this extra blood.
Vascular Resistance
Resistance refers to the factors that oppose the flow of blood.
Resistance is caused by friction as blood moves through a vessel. The resistance to
blood flow as it moves from the aorta to the vena cavae is known as total
peripheral (i.e., systemic) resistance. This is in contrast to the
pulmonary vascular resistance that the right ventricle pumps into. Although
resistance is commonly calculated across the entire circulation, it can also be
calculated for a particular type of vessel in the body (such as the systemic
arterioles), an organ, or a single specific blood vessel, as long as the pressure
drop across the region and the blood flow rate are known. In fact, the previous MAP
equation (2 on previous page) is a special case of this more general equation:
where ΔP is the change in pressure, or pressure drop across the region or vessel of
interest and flow is the flow rate through the same region or vessel. Equation (3)
can be rewritten in the form
Although resistance can be calculated by knowing pressure drop and flow (equation
(4)), resistance of a single vessel can also be determined if we know something about
the physical characteristics of the vessel and blood. There are three factors that
influence vascular resistance: lumen size, blood viscosity, and blood vessel length.
These factors are shown mathematically in the equation, which calculates the
resistance of a single vessel.
Vessel Lumen Size
The smaller the lumen, the greater the resistance. Most of the changes in lumen size
are due to the vasoconstriction and vasodilation of arteries and arterioles. As a
vessel constricts, the lumen size decreases. This increases resistance, which is
inversely proportional to the change in radius to the fourth power.
This means that even small changes in radius can have large affects
on resistance. And, if the flow rate is kept constant when radius decreases, then the
pressure change (drop) along the length of the vessel will increase, as indicated by
equation (3).
Because our systemic vasculature is made up of millions of vessels, they each
contribute in some way to the overall resistance of the system, called systemic
vascular resistance (SVR) or total peripheral resistance. Most of this resistance is
in the smallest vessels, such as small arteries, arterioles, capillaries, and
venules. There is little resistance in large arteries and veins. Because of their
large diameters, only a small amount of blood in large arteries and veins is in
contact with the vessel wall. Controlling SVR is a chief function of arterioles. They
regulate SVR—and thus BP and perfusion, or blood flow, to specific tissues—by
altering their diameter. Even minimal amounts of vasodilation or vasoconstriction in
arterioles can have significant effects on SVR.
Blood Viscosity
Viscosity is a measurement of a fluid's internal resistance (how easily
the fluid particles can slide past each other), which determines its ability to flow.
Viscosity depends on the thickness or stickiness of a fluid. A fluid with a low
viscosity flows quickly; a fluid with a high viscosity flows slowly. When the
viscosity is high, it is hard to maintain fluid movement, and molecules cannot freely
slip past each other. The viscosity of blood is mostly dependent on the ratio of red
blood cells to blood plasma. The more red blood cells in a given volume of blood, the
more viscous the blood. The amount of plasma proteins in the blood also affects
viscosity. If blood viscosity increases, resistance increases proportionally as
indicated by equation (5). If blood flow stays the same, an increased viscosity
requires that the heart generate more pressure to move the flow through the
system.
Blood Vessel Length
There is a direct correlation between the length of a blood vessel and the amount of
resistance (Equation 5). As length increases, so does resistance. This may be obvious
if we consider the pulmonary circulation versus the systemic circulation. Each
circulation receives the same amount of blood flow, but the pulmonary circulation
requires much less pressure to maintain this flow rate. A major reason for this is
that the path length for blood flow is much shorter through the pulmonary circulation
than it is through the systemic circulation.
Resistance to blood flow increases in people who are overweight because even a
kilogram or two of excess fat requires miles of additional small vessels to supply it
with blood. This increased vessel length dramatically increases the amount of
peripheral resistance. A common consequence of obesity is hypertension.
The ultimate goal of the cardiovascular and respiratory systems is to deliver
oxygen-rich and nutrient-rich blood to the capillary networks. Although the amount of blood
within the capillaries at any given time is only about 5 percent of the total
volume, it is the most important blood in the body. It is at the level of the
capillaries that capillary exchange occurs. The thin and porous
construction of capillary walls provides an avenue for substances such as
oxygen and glucose to move out of the blood plasma and into the interstitial
fluids surrounding the tissue cells. The reverse process, uptake of substances
such as carbon dioxide and wastes from the interstitial fluids into the plasma,
occurs simultaneously. In addition to the back-and-forth exchange of solutes,
water also moves between the bloodstream and interstitial fluid in both
directions within the capillary networks. There are three mechanisms by which
substances enter and leave capillaries: diffusion,
transcytosis, and bulk flow.
Diffusion
Diffusion, the movement of a solute from an area of high concentration to
an area of low concentration, is a passive process. Diffusion is the
driving force for the exchange of solutes at the capillary tissue
interface. Thus, the oxygen and glucose, whose concentrations are high in
the blood, will move out of the vessels into the interstitial fluids.
Carbon dioxide levels are high in the interstitial fluid due to diffusion
from the tissue cells. Carbon dioxide will move across the capillary
walls into the blood plasma, where the concentration is lower. The
physical characteristics of each solute determine the route by which that
solute will diffuse. Small water-soluble molecules, such as glucose and
amino acids, diffuse primarily through the intercellular clefts between
the endothelial cells in continuous capillaries or through the
fenestrations of fenestrated capillaries. Lipid-soluble molecules, such
as oxygen and steroid hormones, can diffuse through the plasma membranes
of the endothelial cells. Larger, lipid-insoluble substances must move
down their concentration gradients via transcytosis.
Transcytosis
Transcytosis is a combination of endocytosis and exocytosis that is
necessary when the substance to be transported cannot be exposed to the
cytoplasm of an intervening cell. Transcytosis is important in moving
large lipid-insoluble molecules such as proteins across the capillary
endothelial cells. A protein, for instance albumin or insulin, which is
high in concentration in the blood plasma, will be enclosed in tiny
vesicles formed from the endothelial plasma membrane. This process is
considered pinocytosis because the molecules dissolved in the blood
plasma are taken up randomly along with the fluid. These molecules are
transported within the vesicles across the endothelial cell. At the
opposite plasma membrane surface, the vesicle merges with the membrane,
and the molecules are released into the interstitial fluid. In the
developing fetus, certain plasma proteins and antibodies are moved from
maternal to fetal circulation via transcytosis.
Bulk Flow
Bulk flow is the movement of fluids due to pressure gradients. Fluids
will move en masse from an area of high pressure
to an area of low pressure. While diffusion is most important in the
transfer of larger solutes, bulk flow controls the movement of fluids
plus the ions and other small particles that are dissolved within the
fluid. The movement of fluids from the capillaries into the interstitial
fluid at the arterial end of the capillary bed is called
filtration. The movement of fluids from the interstitial
fluid into the capillaries at the venous end of the capillary bed is
called reabsorption.
Bulk flow follows the same principles as any other type of pressure gradient.
High pressure in one area promotes movement of fluid to an area of lower
pressure. Capillary exchange is driven by two types of pressures. Physical or
hydrostatic pressure is caused by fluid pressing against the
confining vessel walls or other physical barrier. This is the same as the blood
pressure measured within the capillary. Osmotic pressure is caused
by the presence of non-diffusing solutes within the fluid. Nearly all small
molecules, such as ions, freely diffuse across the capillary wall; as such,
they don’t contribute to osmotic pressure differences between plasma and
interstitial space like they do across cell membranes. Instead, it is the large
plasma proteins that are responsible for the osmotic pressure differences
across the capillary wall. The name given to the osmotic pressure caused by
these proteins is colloid osmotic pressure. Depending on the
location, the sum of the hydrostatic and colloid osmotic pressure, called the
net filtration pressure (NFP), can promote filtration or
reabsorption, or all the pressures can be at equilibrium and neither filtration
nor reabsorption occurs. There are four pressures to consider when determining
NFP, two on each side of the capillary wall: blood hydrostatic pressure (BHP),
interstitial fluid hydrostatic pressure (IFHP), blood colloid osmotic pressure
(BCOP), and interstitial fluid colloid osmotic pressure (IFCOP).
Figure 9
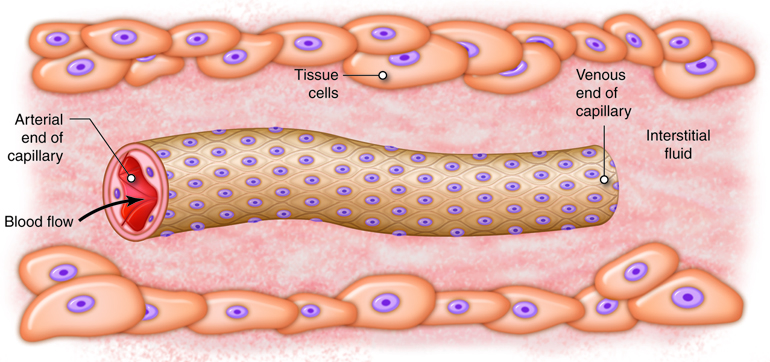
At the arterial end of a capillary network, the BHP is usually about 35 mmHg. A
high BHP promotes fluids moving from the capillaries into the interstitial
fluid around the tissues. Alternatively, IFHP is very low throughout the
tissues surrounding the capillary network. This is because local lymphatic
vessels are constantly removing fluid from the interstitial space, keeping
pressure very low. IFHP is so close to zero that it cannot counter the movement
of fluid into the interstitial fluid under the effects of a high BHP. Due to
this, IFHP is not typically considered in determining NFP. If there was not
another force to counteract the hydrostatic pressure difference across the
capillary wall, there would be a huge outflow of fluid from the capillary into
the interstitial space. In order to provide balance and prevent this from
happening, the high colloid osmotic pressure in the capillaries helps retain
fluid in the vascular space. Most plasma proteins, such as albumin, fibrinogen
and immune globulins are too large to diffuse from the bloodstream except at
sinusoid capillaries. Therefore, the concentration of proteins or colloids is
much greater in the capillaries than in the interstitial fluid. The result of
this imbalance is an osmotic gradient that moves fluid from the interstitial
fluid into the capillaries. The osmotic pressures show little variation along
the length of the capillary network. BCOP holds steady at about 26 mmHg, while
IFCOP is always close to 1 mmHg.
Starling’s law of the capillaries states that the movement of
fluid between the capillaries and interstitial fluid is due to the net effect
of all four of the pressures described. An equation can be used to calculate
the NFP and determine the direction of the fluid movement.
BHP and IFCOP promote the movement of fluid out of the capillaries or
filtration. BCOP and IFHP promote the movement of fluid into the capillaries or
reabsorption. If the pressures that promote filtration are greater than the
pressures that promote reabsorption, the NFP will have a positive value. If the
pressures that promote reabsorption are greater, the NFP will have a negative
value.
Under normal physiological conditions, the values of IFHP, BCOP, and IFCOP do
not significantly vary from the arterial end of the capillaries to the venous
end. Therefore, in most tissues, the rate of filtration and reabsorption is due
to BHP. To demonstrate, assume the following pressures at the arterial end of a
capillary network:
Because the value of NFP is positive, filtration occurs at the arterial end of
the capillary. Moving along the capillary, the BHP becomes lower as blood moves
along the pressure gradient of the vascular system, but the other three
pressures will not fluctuate. At about the midpoint of the capillary network,
the pressures that promote filtration are equal to the pressures that promote
reabsorption. Moving away from the midpoint toward the venous end of the
capillary, the BHP dips below the BCOP. From this point forward, reabsorption
occurs. Near the junction of the capillaries with the venules, the BHP is about
16 mmHg.
This reabsorption value is close to the initial filtration value at the
arterial end of the capillary. This means that most of the water that moved
from the capillaries into the interstitial fluid at the arterial end will be
returned to the bloodstream at the venous end. In fact, about 85 percent of the
fluid filtered from the capillaries is reabsorbed. The remaining 15 percent
flows through the tissues and into the lymphatic capillaries. The lymphatic system
returns the excess fluids plus any plasma proteins that escape back into the
bloodstream. About 22 liters of fluids are filtered from the capillaries each
day. Of this, approximately 19 liters is reabsorbed back into the venous end of
the capillaries, with the lymphatic system picking up the remaining 3 liters of
non-reabsorbed fluid per day.
The accumulation of excess fluid in the tissues is called edema.
There are various physiological causes of edema, including standing too long
without moving your feet and legs, and pregnancy. Edema can also come about as
a side effect of certain medications and can, in some cases, be a sign of a
serious underlying medical condition, such as heart failure. Each cause leads
to an imbalance in the rates of filtration and reabsorption during capillary
exchange. When fluid is filtered from the capillaries more quickly than it can
be reabsorbed, or if the lymphatic capillaries cannot remove normal amounts of
interstitial fluid, the interstitial fluid begins to collect within the
affected tissue or organ. If the volume of the interstitial fluid exceeds 30
percent above normal levels, edema can be detected as swelling of the affected
area.
Most commonly, edema is caused by venous congestion (increased venous
pressures) caused by increased resistance to blood flow through major veins,
reduced pumping ability of the right heart, or excessive fluid levels in the
body. Pregnancy and liver disease can make it harder for blood to return
through the venous system from the legs or abdomen, respectively. In each case
edema will occur in the tissues with impeded flow. Pregnancy commonly causes
edema in the legs, while liver disease causes abdominal edema, called ascites.
Right heart failure leads to a more generalized edema, as venous flow from the
entire body is affected. In each case, BHP is greatly increased within the
capillaries. Because BHP is the driving force of filtration, the rate of fluid
leaving the capillaries escalates with increasing BHP. High BHP also opposes
reabsorption. Despite the rise in IFHP due to extra fluid within the tissues,
the high BHP results in less fluid reabsorption at the venous end of the
capillary network. A high BHP also puts uncommon pressure on the walls of the
capillaries, forcing the epithelial cells apart, and the intercellular gaps
become wider. Plasma proteins normally confined to the blood vessels spill into
the interstitial fluid. This causes IFOP to increase and BCOP to decrease,
causing fluid to accumulate even more rapidly. Edema can only be resolved by
intervention to prevent the filtration/reabsorption imbalance and time for the
lymph system to collect the excess fluids.
The volume of blood in the systemic veins that is flowing back to the heart is called
venous return. Blood returns to the heart through veins because of the
difference in pressure between venules (approximately 16 mmHg, on average) and the right
ventricle (0 mmHg). Any pressure increase in the right ventricle or right atrium lessens
venous return. A leaky ("incompetent") tricuspid valve raises right atrial pressure,
because it allows blood to flow backward ("regurgitate") when the ventricles contract. The
resulting reduction in venous return is accompanied by an accumulation of blood in veins of
the systemic circulation.
In the upright individual, return of venous blood from the lower body is particularly
challenged. This is because the flow of blood has to overcome the effects of gravity as
well as the normal resistance of the vessels. Therefore, two additional
factors help force blood toward the heart: the skeletal muscle pump and the respiratory
pump. Both mechanisms rely on venous valves to operate.
Skeletal Muscle Pump
The skeletal muscle pump uses muscle contractions in the legs to force
venous blood upwards toward the heart. The peripheral veins in the legs have one-way
valves that route blood away from the legs and toward the heart. When standing still,
these valves prevent the backflow of blood that might otherwise occur due to
gravitational pull. When muscles of the legs contract, they tend to “squeeze” the
veins of the legs; propelling blood. Because of the one-way valves, blood will only
move toward the heart, helping maintain venous return. Lack of use of this skeletal
muscle pump contributes to the lower leg edema common in people who are bedridden or
confined to a wheelchair. When flow in the legs is not maintained, the venous system
there becomes congested. This leads to increased capillary hydrostatic pressure and
resultant edema.
Respiratory Pump
Sequential compression and decompression of veins also drives the respiratory
pump, which operates on abdominal and thoracic veins. When you inhale,
downward movement of the diaphragm reduces the pressure in the thoracic cavity and
increases the pressure in the abdominal cavity. The resulting compression of
abdominal veins pushes blood into the decompressed thoracic veins and then into the
right atrium. When you exhale, the pressures reverse, and the one-way valves in the
veins prevent blood from flowing back to the abdominal veins from the thoracic
veins.
The two pumps of the heart are in series, meaning that the blood flows in one direction
from one pump to the other. Starting from the left heart, the blood flows through the
peripheral circulatory system, which provides the body with the nutrients it needs, such as
oxygen, and removes metabolic waste, such as carbon dioxide. As blood moves through this
circulation, it follows a path of blood vessels that change in their anatomy and function.
Upon leaving the heart blood enters the major arteries. Arteries are the
vessels in the circulatory system that carry blood away from the heart. These arteries
branch and divide into arterioles that are smaller in diameter. These
arterioles continue to branch out to create even smaller capillaries. These
capillaries, which are found within each organ, are the site of gas and nutrient exchange.
Once blood has perfused the organ, the capillaries begin to coalesce into larger
venules that combine to create larger veins, ultimately returning blood to
the heart through either the superior or inferior vena cava.
Circulatory Pathways
Blood vessels are organized into circulatory routes that deliver blood
to different organs and tissues of the body. The right ventricle pumps blood to the
lungs via the pulmonary circuit. The left ventricle pumps blood to
organs and tissues via the systemic circuit. The output of the systemic
circuit becomes the input of the pulmonary circuit, and vice versa. The pump for the
systemic circuit is the left side of the heart, which also collects the oxygen-rich
blood returning from the lungs. The pump for the pulmonary circuit is the right side
of the heart, which receives oxygen-poor- blood from the systemic circulation.
There are two important differences between the systemic circulation and pulmonary
circulation. The first is that blood in the systemic circulation must be pumped
farther than blood in the pulmonary circulation. The second is that, in pulmonary
arteries, the diameter is larger, the walls are thinner, and the tissue is more
elastic than in systemic arteries. Because of the characteristics of pulmonary
arteries, there is very low resistance to blood flow in the pulmonary circuit, and
thus pressures do not need to be very high to move blood through the lungs. In the
right ventricle, the peak systolic pressure is typically one-fifth that in the left
ventricle.
The Pulmonary Circuit
The sole function of the pulmonary circuit is to carry blood to the alveoli of the
lungs so that gases can be exchanged. Flow is matched to ventilation so that oxygen
can move from the alveoli into the bloodstream and carbon dioxide can move from the
bloodstream into the alveoli. The pulmonary circulation begins at the right
ventricle, which pumps oxygen-poor, carbon dioxide-rich blood into the large
pulmonary trunk. From its origin at the right ventricle, the pulmonary
trunk takes an upward diagonal course for approximately 8 cm (3.1 inches
(in.)). It then divides into the right pulmonary artery (to the right
lung) and the left pulmonary artery (to the left lung). With the
exception of the fetal circulation, the pulmonary arteries are the only arteries that
transport oxygen-poor blood. After entering the lungs, the pulmonary arteries
subdivide into three lobar arteries in the right lung and two lobar
arteries in the left lung. Each lobar artery supplies one lung lobe. Further
subdivisions result in the formation of capillaries around the alveoli. Carbon
dioxide from the blood is transferred into the alveoli and exhaled. Inhaled oxygen is
transferred from air in the lungs into the blood.
Pulmonary capillaries merge, forming venules that unite to become pulmonary
veins. Two of these veins leave each lung and transport oxygenated blood to
the left side of the heart. As the left ventricle contracts, it pumps out the
oxygenated blood into the systemic circulation. Again, except for fetal circulation,
pulmonary veins are the only veins that transport oxygen-rich blood. It is easy to
remember which vessels belong to the pulmonary circulation, because they all have
either the word pulmonary or lobarin
their names.
The Systemic Circuit
The systemic circuit delivers oxygenated blood and nutrients to the entire body
except the lungs, and removes the waste products of metabolism from the same tissues.
We will review the circuit from previous sections: After blood is pumped from the
left ventricle into the aorta, it flows into smaller and smaller systemic arteries to
perfuse all of the body's organs and tissues (except for the alveoli of the lungs).
Systemic arteries branch off into smaller arterioles that lead into extensive
networks of systemic capillaries. Nutrients and gases are exchanged between blood and
systemic tissues through the thin capillary walls. After the blood gets rid of oxygen
and picks up carbon dioxide, it usually travels through a single capillary before
entering a systemic venule. The venules then transport oxygen-poor (deoxygenated)
blood from systemic tissues before merging to create the larger systemic veins. All
blood in the systemic circulation eventually empties into the right atrium.
The arteries and arterioles of the systemic circulation receive oxygenated blood from
the left ventricle and transport it to the systemic capillaries. The veins and
venules of the systemic circulation carry deoxygenated blood back to the right
atrium. Blood in systemic arteries is bright red in color. Blood in capillaries is
dark red, because it has lost some oxygen and gained carbon dioxide.
The arteries and veins of the systemic circuit share a number of similarities, most
notably, the routes and names that many of these vessels share. The blood from the
heart is pumped into a single systemic artery, the aorta, which carries oxygenated
blood away from the left ventricle. Oxygen-poor blood returns to the
right atrium via three systemic veins: the superior vena cava, inferior vena cava,
and coronary sinus. The majority of blood that returns to the heart flows through the
superior and inferior venae cavae. The names of these veins are derived from the
location of their tributaries. The superior vena cava collects blood
from veins located superior to (above) the diaphragm, with the exception of the wall
of the heart and the alveoli of the lungs. The superior vena cava, which delivers
blood to the right atrium, is formed by the merger of the right and left
brachiocephalic veins. Each of these veins, in turn, is formed by the
union of the internal jugular vein and subclavian vein on
the right or left side of the body. The inferior vena cava, which is the
largest-diameter blood vessel in the body, collects blood from veins located inferior
to (below) the diaphragm and delivers it to the right atrium. This vein is located
directly to the right of the abdominal aorta. Its distal end is created by the merger
of the paired common iliac veins. Blood that drains from the myocardium
of the heart empties into cardiac veins and returns to the right atrium via the
coronary sinus.
Overview of Blood
The adult human body contains approximately 5 liters of blood (8 percent of the
body’s weight), which courses through the heart, arteries, veins, and
capillaries.
As
blood courses through blood vessels it carries out essential functions in the
body, such as transporting nutrients, gases, hormones, and heat toward the
body’s tissues and carrying waste material, carbon dioxide and excess heat away
from these same tissues. The blood also plays an important role in protecting
the body against microbial infection and forming clots to protect against blood
loss. It takes about 60 seconds for blood to travel completely through the body
and return back to the heart.
Blood Properties: color, pH, specific gravity and viscosity
Arterial blood that flows into the aorta from the left ventricle is fully
oxygenated so that it can deliver the oxygen necessary for the tissues to make
adequate ATP. The combination of oxygen bound to the heme groups in the red
blood cells gives this blood a red pigment. Venous blood, which returns to the
right side of the heart after having exchanged nutrients in the tissues, appears
darker in color because it contains a larger fraction of hemoglobin that is no
longer combined with oxygen. In the pulmonary circulation, it is the pulmonary
artery blood that is less oxygenated, and therefore darker in color in compared
to the brighter red blood in the pulmonary
veins.
The
heme groups in the red blood cells are part of larger hemoglobin molecules,
which are found in high concentration within red blood cells (RBCs). These
oxygen carrying cells typically make up 35-45% of blood volume. Together with a
small fraction of white blood cells (WBCs) and platelets, they
collectively make up the formed elements of the blood. The rest of the blood is
composed of a fluid fraction called plasma. Plasma is composed of
water and other substances such as proteins, salts, and gases. The pH of blood
plasma ranges from 7.35 to 7.45. It has a specific gravity between 1.050 and
1.060 in adults. Specific gravity is a measure of blood density related to pure
water (water has a specific gravity of 1.000), so blood is about 5-6% more dense
than water. The specific gravity of blood may vary and can be affected by
certain diseases that change the concentration of cells within the plasma.
Besides specific gravity, viscosity is another fluid property that
may be of importance in blood. Viscosity is the internal friction liquid
exhibits when it tries to flow. For example, honey and syrup have a much higher
viscosity than water, and they flow slower under similar conditions than water
would. The viscosity of pure water is 1.00, whereas the viscosity of blood can
be roughly three to five times that because it contains cells and proteins.
Blood helps in the regulation of body temperature. The blood entering the body’s
core (the area in which thoracic and abdominal organs are located) is
immediately warmed to a temperature of approximately 38°C (100.4°F). Depending
on body temperature, this blood may end up traveling to the skin where it can
transfer some of this heat to the external
environment.
Overview of Plasma
Plasma is the fluid portion of blood with no cells.
Serum is the portion of plasma that does not contain any
blood-clotting proteins. Plasma consists of mainly water (90 percent). Other
substances found in plasma include proteins, respiratory gases (O2
and CO2), electrolytes (sodium, chloride, calcium, and
bicarbonate), nutrients, and waste products (e.g., urea and uric acid). The most
abundant of the proteins is albumin, which helps retain water in the vascular
compartment. The function of plasma is to transport dissolved nutrients and
gases, regulate pH, and contribute to fluid and electrolyte balance.
Plasma Proteins
Plasma proteins, which make up approximately 7 to 9 percent of plasma, consist of
albumin, globulins, and fibrinogen. These molecules carry out several important
functions within the body. Albumin is the smallest, yet it accounts
for the majority of these plasma proteins (60 percent). Albumin is synthesized
by the liver and functions to maintain colloid osmotic pressure in the blood
compartment. This helps prevent the leakage of fluid into the surrounding
interstitial spaces, and helps prevent edema (swelling). Three
types of globulins make up about 36 percent of the plasma proteins:
alpha, beta, and gamma. The alpha and beta globulins transport lipids and metals
in blood. Gamma globulins are the antibodies released by immune
cells that attack microorganisms and other foreign molecules. Fibrinogen makes
up about 4 percent of plasma proteins and is important in the formation of blood
clots.
Plasma Proteins and their Functions
| Protein |
Site of Origin |
Function |
Percentage of Plasma |
| Albumin |
Liver |
Maintains colloid osmotic pressure and contributes to the viscosity of
blood |
60 |
| Globulins: |
|
|
|
| Alpha |
Liver |
Transport lipids and fat-soluble vitamins |
36 |
| Beta |
Liver |
Transport lipids and other molecules |
|
| Gamma |
Lymphocytes |
Immunity |
|
| Fibrinogen |
Liver |
Contributes to blood clotting |
4 |
Disorders of Blood Plasma: Hypoalbuminemia
Albumin is a transport protein that carries various small molecules, such as
bilirubin, metals, hormones, and medications, through blood. Another important
function of albumin is to prevent plasma from leaking into tissues. If albumin
levels are low, fluid will leak into the tissues, resulting in edema (swelling).
Serum albumin levels typically decrease secondary to liver disease (including
hepatitis and cirrhosis), loss of albumin in the urine, poor nutrition, or
gastrointestinal disorders (celiac, Crohn’s and Whipple’s disease) that
interrupt normal nutrient absorption. Levels may be increased with increased
concentration of hormones such as steroids, growth hormone or insulin. The
levels of albumin can be measured with a serum albumin test; with normal levels
between 3.4 and 5.4 g/dL.
A consequence associated with hypoalbuminemia is that medications that are
carried by albumin may have an increased concentration of unbound drug in the
plasma. It is the unbound fraction that is active, such that even normal doses
may seem like an “overdose”. Hypoalbuminemia occurs in acute and chronic
inflammatory responses. As a result, a serum albumin plays an important
prognostic role among hospitalized patients; as it is correlated with an
increase risk of morbidity and mortality. Hypoalbuminemia commonly occurs among
older adults who live in long-term care facilities, hospitalized patients with
chronic illnesses, and malnourished children.
Hemostasis
Hemostasis (hemo- blood and stasis – standing still) is a protective
process in which the body undergoes a cascade of steps in order to stop the
bleeding from a damaged blood vessel. The process of hemostasis is incredibly
complex so that blood clots form where and when we need them to. We have all
seen that small cuts on our skin begin the healing process with a clot. However,
clots are not always beneficial. The formation of blood clots within an intact
blood vessel can lead to heart attacks and strokes. Thus, this process has to be
well regulated. Hemostasis is a multi-step process, characterized by three major
activities: vasoconstriction, platelet aggregation, and formation of a fibrin
clot.
When a blood vessel is torn, the first response is a vascular (vascular refers to
blood vessels) spasm, which is the constriction of the blood vessel at its site
of injury. This vascular constriction is initiated by activation of pain
receptors that reflexively cause constriction of local smooth muscle cells.
Second, platelets (a type of cell fragment) stick to the collagen
fibers that become exposed when the endothelial cell lining of the blood vessels
is damaged during injury. In doing so, they begin to plug up the hole. These
bound platelets also become activated and release secretory granules that
contain adenosine diphosphate (ADP), serotonin, prostaglandins, and
phospholipids. These signaling molecules lead to binding of more platelets at
the site of injury. This process continues until enough platelets have
aggregated to form a platelet plug. Serotonin, a neurotransmitter,
and thromboxane A2, a prostaglandin produced by activated
platelets, both facilitate vasoconstriction at the site. The released
phospholipids activate the clotting factors. In the end, the platelets at the
site of injury form of loose meshwork of interconnected platelets.
Third, a more stable plug called a clot, or thrombus, forms via
coagulation (blood clotting). Coagulation occurs as a series of
reactions that result in the formation of the fibrin protein. This fibrin
protein can cross-link to other fibrin proteins, as well as to platelets,
providing strength to the thrombus. The end product of coagulation is a clot, or
thrombus, consisting of a fibrinous gel that encloses trapped blood cells.
During the coagulation process the clotting cascade amplifies itself, making
this a classic example of a homeostatic feedback loop that uses positive
feedback (although in a limited manner).
Figure 1
Fibrin is formed from the plasma protein fibrinogen. This conversion
occurs only after a cascade of enzymatic reactions involving various plasma
proteins called clotting factors. The 13 clotting factors are named
in the order of their discovery as I through XIII. Two separate pathways exist
in the clotting pathway: an extrinsic and intrinsic pathway. The majority of the
reactions that occur in both the extrinsic and intrinsic pathways require
calcium in order for the steps to occur. Coagulation can be inhibited by adding
a chelating agent, such as citrate, that binds to calcium making it
unavailable to facilitate blood clotting. This is a way to prevent freshly drawn
blood from clotting.
Intrinsic Pathway
The intrinsic pathway is activated when plasma is exposed to
collagen after endothelial cell injury, or when blood is exposed to negatively
charged surfaces such as glass. For example, if blood is added to a test tube
that doesn’t contain any clot inhibiting chemicals, the intrinsic pathway will
be activated, causing clots to form. The intrinsic pathway starts with the
activation of factor XII (a protein-digesting enzyme), which removes the
inactive part of factor XI in order to activate it. Activated factor XI, in
turn, activates factor IX. Phospholipids (provided by platelets) and calcium are
utilized in the remainder of the steps in the cascade. A complex is formed with
phospholipids, calcium, factor IX and factor VIII. This new complex activates
factor X, and they all combine to convert prothrombin into thrombin followed by
the conversion of fibrinogen into fibrin. Fibrin polymerizes to form long fibers
that interconnect and form the meshwork of the clot. Platelets are interweaved
into the fibrin mesh and further fill the clot.
Extrinsic Pathway
The extrinsic pathway is triggered by chemicals released by the
damaged cells of the blood vessel wall. In this pathway, clot formation occurs
rapidly with the release of tissue thromboplastin (factor III), by the damaged
tissue cells. Factor III activates another clotting factor called factor VII.
Phospholipids and calcium are added to factor VII in order to form a complex
(VII complex) that in turn activates factor X. Factor X, factor V,
phospholipids, and Ca++ all contribute to the formation of the V
complex that converts prothrombin (inactive factor II) into thrombin. Thrombin
then converts fibrinogen (factor I) to fibrin. Fibrin is acted on by factor XIII
to form an impenetrable fibrin clot.
Figure 2
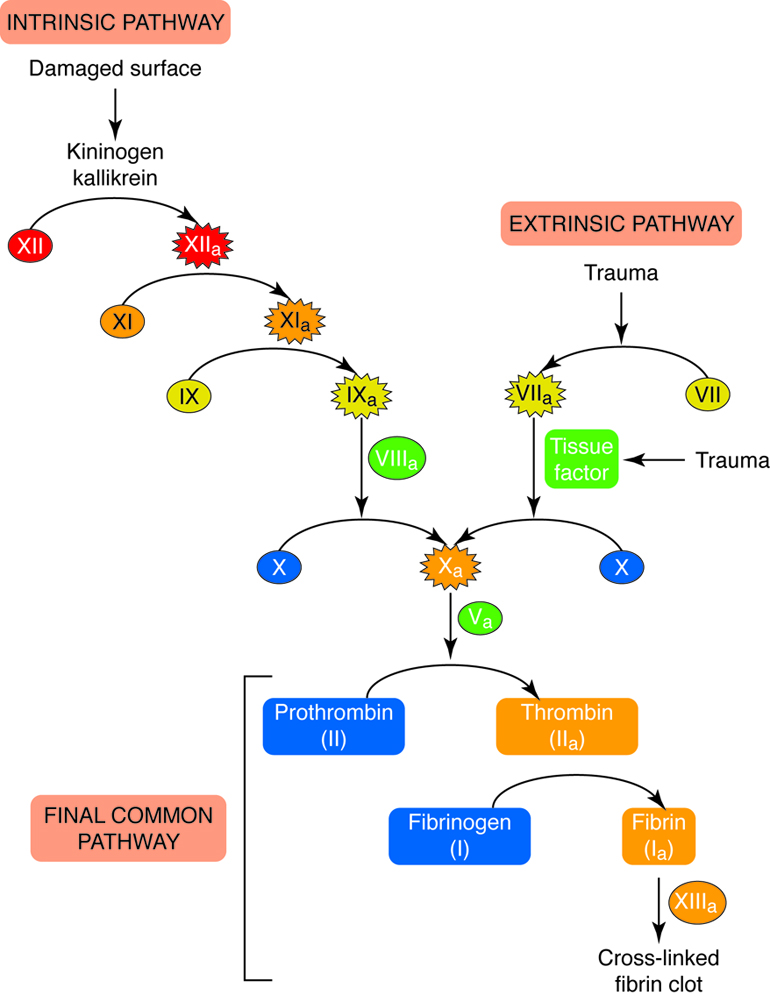
Fibrinolysis
After the clot has performed its function of stopping blood loss, the
constituents of the clot have to be removed. Clot retraction is the
gradual removal of the fibrin threads that make up the blood clot, and
fibrinolysis is the process by which a blood clot is broken
down. Once a damaged blood vessel wall is undergoing repair, factor XII will
convert inactive prekallikrein into active kallikrein. Kallikrein
is responsible for the conversion of an inactive plasma protein called
plasminogen into plasmin. Plasmin is an
enzymatically active molecule that splits the fibrin polymers that make up the
clot. Kallikrein also plays a role in the production of bradykinin,
which stimulates vasodilation to help counter the vasoconstrictive effects of
serotonin and prostaglandins that were released by platelets during clot
formation.
Problems in Blood Clotting
Normal hemostasis can be reduced by a number of conditions, including decreased
platelet levels, genetic defects leading to insufficient or abnormal clotting
factors (hemophilias), or lack of production of clotting factors in the liver
(where many are formed). Certain clotting factors require vitamin
K, a fat-soluble nutrient that is synthesized by bacteria in the large
intestine. Conditions that result in the malabsorption of fat lead to the
deficiency of vitamin K and correspondingly increased bleeding times.
Blood clots can break free from sites of injury. These
thromboemboli then get trapped within small blood vessels, blocking flow
through the vessels. Thromboemboli that originate in the venous circulation
travel until they reach the small blood vessels in the lungs where they lodge as
pulmonary emboli. If these thromboemboli are large enough, the person will have
abnormal breathing or may die.
Sometimes it is beneficial to inhibit the clotting cascade to lower the risk of
thromboemboli. During surgery this is usually done with the injectable drug
heparin, which rapidly inactivates the enzyme thrombin. Heparin is used because
it can be rapidly reversed. For those that need a longer term reduction in
clotting ability, the oral drug Warfarin is commonly used. This drug is a
vitamin K antagonist and reduces the levels of several clotting factors.
There are three main types of blood cells that flow through the blood stream, with each
type serving specialized functions. Red blood cells primarily carry oxygen and remove
carbon dioxide, white blood cells are immune cells that patrol the blood and body
tissues for pathogens and platelets, which are involved in hemostasis, as described
previously.
Figure 3
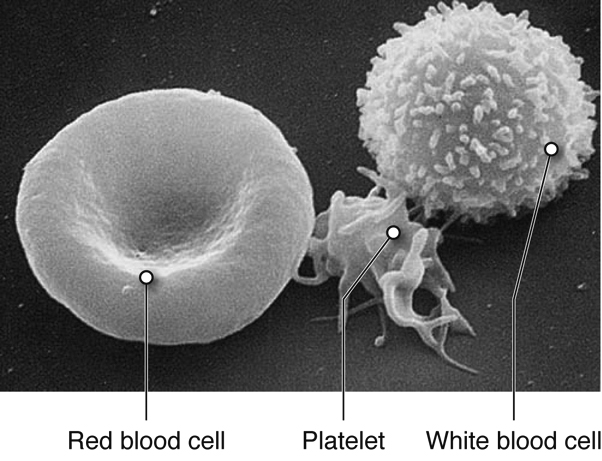
Figure 4
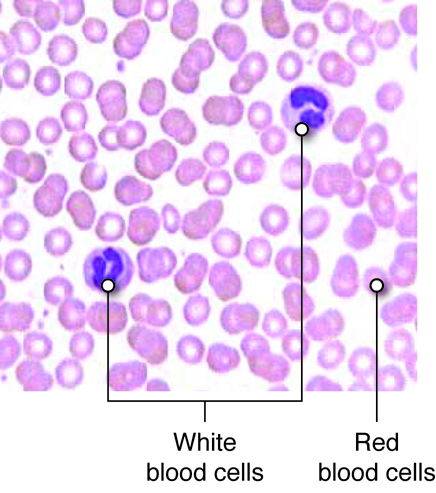
Red blood cells (RBCs) are one of the most abundant formed elements in blood. Also called
erythrocytes, red blood cells are specialized for oxygen transport and function
primarily in gas exchange. Red blood cells bring oxygen to tissues and pick up carbon
dioxide (the gaseous waste product of cells), bringing it back to the lungs. Red blood
cells contain hemoglobin, a protein that contains iron, to which both oxygen and carbon
dioxide are able to bind. In the lungs, red blood cells pick up the oxygen that they
transport.
The various types of white blood cells (WBCs), or leukocytes, are readily distinguished
by whether or not they possess granules within their cytoplasm. Granulocytes, as their
name suggests, possess numerous granules in their cytoplasm, whereas agranulocytes do
not. The shapes of the nuclei of granulocytes and agranulocytes also differ. Leukocytes
mainly function to protect the body from microbial invasion and toxins. WBCs readily
travel through the bloodstream. However, most leukocytes can also travel from the bloodstream
into the interstitial tissues in order to attack foreign agents in those spaces.
Platelets are smaller fragments of larger cells called megakaryocytes, and they are
sometimes not even classified as cells. Megakaryocytes are large nucleated cells off of
which numerous platelets bud. Platelets lack a nucleus but do have a plasma membrane
surrounding a cytoplasm and a few organelles. As described previously, platelets play a
role in hemostasis.
Red blood cells (RBCs), also called erythrocytes (erythro- red and cytes –cells), are disc
like cells that are the most abundant cell types in blood. Red blood cells transport the
oxygen from our lungs to all of our body’s cells that carry out aerobic respiration.
RBCs also pick up carbon dioxide (the gaseous waste product of cells) from metabolically
active cells, bringing it back to the lungs. Red blood cells contain a special protein
that contains iron, called hemoglobin (Hbg), to which both oxygen and
carbon dioxide are able to bind. Red blood cells do not have nuclei or many other
organelles, which leaves more room in cytoplasm for hemoglobin needed for the cell's job
to transport these molecules.
Figure 5
Hemoglobin consists of four heme groups (non-protein) and four globin
(protein) molecules. Each heme group is composed of an iron structure surrounded by
carbon rings containing nitrogen. Heme, in combination with oxygen, is responsible for
the red pigment of blood. Each hemoglobin molecule can transport up to four oxygen
atoms, one bound to each heme group, when the hemoglobin is said to be fully saturated.
In fetuses and infants, hemoglobin is mainly composed of two alpha and two gamma globin
chains (there are very few beta chains). As an infant develops, the gamma chains are
replaced by beta globin chains. In an adult, hemoglobin consists of two alpha and two
beta globin chains. A single erythrocyte has about a quarter of a billion hemoglobin
molecules, which demonstrates the amazing capacity of red blood cells to transport
oxygen.
Figure 6
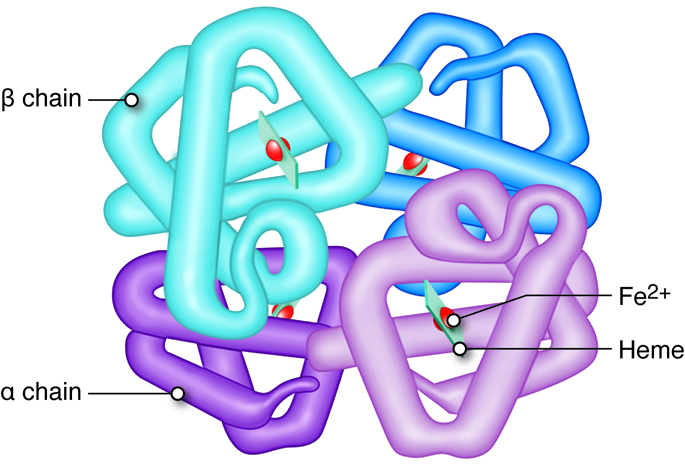
|
Figure 7
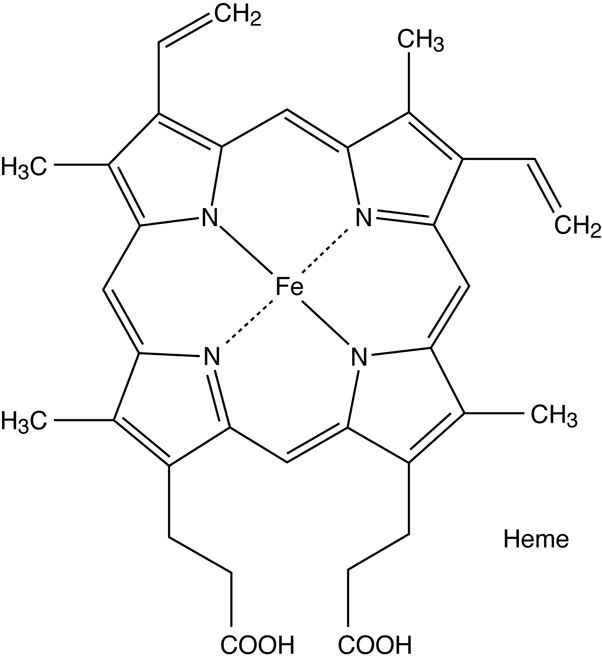
|
Blood passing through the lungs is exposed to high oxygen levels, and when red blood
cells pick up this oxygen the hemoglobin is called oxyhemoglobin
(HbO2). As blood passes through other tissues, oxygen-carrying
RBCs drop off a fraction of this oxygen (typically 25%). The hemoglobins that are no
longer carrying oxygen are called deoxyhemoglobin (Hb).
If hemoglobin has lost a fraction of its bound oxygen, then a small amount of carbon
dioxide can bind to the protein sections of Hb (versus oxygen that binds to the heme),
and can be transported from tissues to the lungs. This hemoglobin is referred to as
carbaminohemoglobin (HbCO2), which accounts for 25 percent
of the carbon dioxide transported in in the blood. However, the bulk of carbon dioxide
collected from tissues throughout the body is transported in the form of
bicarbonate (HCO3-) in the plasma. The enzyme
carbonic anhydrase, found in high concentration in erythrocytes,
catalyzes the conversion of carbon dioxide and water into bicarbonate. This bicarbonate
then moves into the plasma in an exchange with chloride across the RBC membrane.
Life cycle of Red Blood Cells
Erythropoiesis is the process by which bone marrow stem cells
develop into erythroblasts that differentiate further into reticulocytes, and
then mature before being released into the circulation (Figure 8). This process
of making RBCs occurs continuously and at a high rate, with approximately 2.5
million RBCs produced every second. This matches the breakdown rate of RBCs, as
old ones are removed and destroyed by the liver and spleen. Erythropoietin is
the hormone from the liver and kidney that regulates the rate of RBC
production.
Mature red blood cells are disc-shaped structures with a flattened center. They
do not possess a nucleus or other organelles, but instead use the space for
hemoglobin. The depressed center also maximizes the surface area of red blood
cells, which facilitates the exchange of O2 and CO2. The
flattened shape of RBCs allows them to navigate through narrow capillaries,
which are often of similar (or smaller) diameter than the RBCs. The lack of a
nucleus also means that RBCs lack the genetic material (DNA) necessary to
reproduce, grow, and carry out functions of maintenance and repair. This limits
the lifespan of these cells.
Figure 8
Red blood cells have a typical lifespan of 120 days. Over time, red blood cells
accumulate cellular damage. Once they get to a point where they cannot deform
appropriately when moving through narrow channels, they are removed from the
circulation by specialized cells, called macrophages, in the spleen or liver.
Macrophages phagocytose (engulf) the old RBCs, separating the
protein (globin) and non-protein (heme) portions of hemoglobin. The protein
chains are further degraded into amino acids and dispersed within the blood
plasma. Iron is extracted from heme and is either stored or enters the
bloodstream. Stored iron is attached to proteins ferritin and
hemosiderin, preventing the buildup of toxic free iron. Iron
entering the bloodstream is also attached to a molecule called a
transferrin; which can be picked up for use by various tissue
types such as muscle cells, or even bone marrow, where it is used to generate
new erythrocytes.
Figure 9
Blood Groups
You are probably aware that if an individual is transfused with non-compatible
blood that negative consequence, such as the clumping together (agglutination)
or destruction (hemolysis), of the donated red cells might result. Compatibility
is determined by the lack of interaction between antigens on the donor red blood
cells and antibodies in the plasma of the recipient. People of different blood
groups (types) vary in the red blood cell antigens and plasma antibodies they
have.
Although there are many blood groups, the ones that are the most important when
it comes to transfusions are the ABO and Rh groups. These groups are genetically
determined, with a single gene coding for protein synthesis being responsible
for each. The alleles inherited determine which if any protein antigen(s)
(sometimes called agglutinogens) are expressed and embedded in the RBC membrane.
In the case of the ABO blood group, the two alleles of the gene can each be one
of 3 different variants, O (i), A (IA), or B (IB). The A and B alleles are
dominant to the O allele, while the A and B alleles (AB or IAIB genotype) are
codominant, creating the AB blood group when inherited together. The O or null
allele results in no antigen being created (so A blood group could be either AA
or AO genotype). For the Rh group the dominant D allele determines if a person
is Rh+ with possible genotypes of either DD, Dd, or dd.
Note that these two blood groups are distinct from one another such that they are
used in combination, and not one or the other. For example, those with blood
type O- express neither antigen A or B or D, while those with type A+ blood
express the A antigen and the D antigen on their red cells.
Besides the antigens, the ABO blood group also determines the antibodies
(sometimes called agglutinins) that are present in the person’s plasma. These
antibodies start forming soon after birth, likely in response to bacteria with
similar membrane antigens that start inhabiting the gut. Those with type O blood
will make both anti-A and anti-B antibodies, those with type AB blood will not
produce either antibody, while those with either type A or type B blood will
make antibodies against the antigen not found on their red cells. In contrast,
those that are Rh-, do not make anti-D antibodies unless exposed to a
significant amount of blood containing the D antigen.
As mentioned, in order for a blood transfusion to be compatible, the donor blood
cannot contain antigens that will cross react with recipient plasma antibodies.
The most obvious way to prevent transfusion reactions is to infuse donor red
blood cells (which is more common than using whole blood) that are of the same
type as the recipients. But in some cases other red blood cells can be used as
well. For example, because those individuals who are blood type AB do not have
any anti-A or anti-B antibodies in their plasma, they can accept blood from any
other ABO blood type (as long as the RH factor matches as well), making them the
universal acceptor.
Anemia
Anemia is a condition in which the oxygen carrying capacity of the blood is
reduced. It is indicated by a low hemoglobin concentration, and typically
presents with a decrease in the number of red blood cells.
Example Forms, Causes, and Clinical Features of Anemia
| Types of Anemia |
Clinical Features |
Origin of Anemia |
| Iron deficiency anemia |
Failure to synthesize adequate amounts of RBCs |
Poor dietary intake of iron, blood loss |
| Pernicious anemia |
Failure to synthesize adequate amounts of RBCs |
Poor intestinal absorption of B12, a vitamin necessary for RBC
production |
| Folic acid deficiency anemia |
Failure to synthesize adequate amounts of RBCs |
Poor intake of dietary folic acid; inability to absorb folic acid;
medications (metformin is a diabetic drug that can interferes with folic
acid utilization) |
| Hemolytic anemia |
Rapid degradation of RBCs |
Genetic or acquired diseases that alter the shape of RBCs; toxins or
drugs |
| Sickle cell anemia |
A form of hemolytic anemia in which RBCs are degraded after they assume
a sickled shape. When sickled, RBCs clump together and block blood
vessels. |
Inherited disease in which RBCs develop a sickle shape;most common among
African Americans |
| Thalassemias |
A form of hemolytic anemia resulting from a family of disorders
associated with abnormal hemoglobin. There are several variants of the
disease depending on if it is the alpha or beta chains that are
affected, and whether the individual is homozygous or heterozygous for
an abnormal gene. |
Inherited diseases that are most common in individuals from Greek,
Italian, Asian, African or Middle Eastern descent |
White blood cells, or leukocytes, are immune cells that are
present in the blood. The detailed mechanisms of immune function are covered in the
immunity unit, but we will discuss the classes here. There are five common types of
leukocytes (and some of those types have subgroups). One way of categorizing these five
is by whether or not they contain granules in their cytoplasm when the cells are
stained. If they do, they are a type of granulocyte. If not, they fall into the category
of agranulocytes. Another way of distinguishing leukocytes from one another is by the
morphology (shape and patten) of their nuclei. Granulocytes have multilobulated nuclei
and agranulocytes have a spherical (nonlobulated) nucleus. The lifespan of a granulocyte
ranges from about 12 hours to 4 days, but the agranulocyte survives for approximately
100 to 300 days.
Leukocytes mainly function to protect the body from microbial invasion and toxins. WBCs
readily travel through the bloodstream. However, certain leukocytes have the ability to
move into the interstitial spaces of the body’s tissues in order to attack foreign
agents to protect the body from infection. They leave the capillalry by squeezing
through the pores in the capillary walls via a process called diapedesis.
This is due to the ability of these leukocytes to rearrange cytoskeletal matrix in a
manner that changes their shape.
Granulocytes
Granulocytes contain many granules within the cytoplasm, and they have a
multilobular, irregularly shaped nucleus. There are three types of granulocytes:
neutrophils, eosinophils, and basophils. Each granulocyte is identified by
specific stains used to reveal the granules in their cytoplasm. Granulocytes are
responders of the nonspecific immune system, which means that the cells respond
to injury and build up an inflammatory response against microbes or toxins
regardless of whether the body has been previously invaded by the foreign agent.
That is to say, granulocytes do not possess memory when mounting an immune
response.
Nonspecific Immune Response Leukocytes
| Neutrophil |
|
|
| Eosinophil |
|
|
| Basophil |
|
|
| Monocyte |
|
|
Neutrophils
Neutrophils are the most abundant type of granulocyte (50 to 70
percent of circulating WBCs). The S- or C-shaped nucleus of these cells has
three to six lobules that are connected via strands of chromatin. The
multi-lobular shape of the nucleus often classifies neutrophils as
polymorphonuclear leukocytes (PMNs).
During a bacterial infection, neutrophils are the first responders. The PMNs
arrive at the site of infection through a process known as
chemotaxis in which chemicals released by damaged cells draw
neutrophils. The neutrophils attack bacteria by engulfing them via the process
of phagocytosis. Once the bacteria are contained within the neutrophils, the
granules release antimicrobial compounds called defensins and
lysozymes to destroy the microbes. Pus is the culmination of
destroyed neutrophils, bacteria, and dead tissue.
Eosinophils
Eosinophils only possess a bilobed nucleus linked by a thin strand
of chromatin. Unlike neutrophils, eosinophils stain red with an eosin dye; the
dye is acidic and is picked up by the cytoplasmic granules that contain
digestive enzymes. Eosinophils also engulf foreign molecules attacking the body,
particularly in parasitic infection and allergic reactions. They also play a
role in modulating the activity of basophils.
Basophils
The nucleus of basophils can contain two to five U- or S-shaped
lobules. These leukocytes pick up basic dyes that cause their granules to have a
blue-purple appearance. The granules of basophils contain histamine, serotonin,
and heparin. The cells release histamine during an inflammatory response to
tissue damage and microbial invasion. Basophils are similar in appearance and
function with mast cells, which are strictly localized in connective tissue.
Agranulocytes
Unlike granulocytes, agranulocytes do not have granules within their
cytoplasm and lack a lobulated nucleus. Under a microscope agranulocytes are
observed to have a nucleus that composes the bulk of its cellular volume.
Agranulocytes include lymphocytes, which are responsible for the specific immune
response, meaning that they have memory and build up a vigorous response against
toxins or microorganisms that the body has encountered before. The other
agranulocyte is the monocyte, which is an immature form of the non-specific
macrophage.
Lymphocytes
Lymphocytes can be categorized by their size (small, medium, and
large). These cells have a round nucleus that virtually fills up the cell
leaving very little blue-staining cytoplasm surrounding it (when stained in a
standard manner). Lymphocytes are the only WBCs that reenter the bloodstream
after moving into tissue to attack foreign agents.
There are two types of lymphocytes: T lymphocytes (T cells) and
B lymphocytes (B cells).
They both originate in the bone marrow, but T cells grow and mature within the
thymus gland while B cells typically mature in bone marrow then move to lymph
nodes and similar tissue. T cells attack abnormal cells in the body, such as
virally infected cells, tumor cells, and donor transplant cells. B cells sense
and attack invading antigens (foreign substance in the body) such as toxins,
viruses, or bacteria. In response to antigens, B cells divide into plasma cells
that make antibodies aimed at neutralizing and destroying these antigens.
Monocytes
The nuclei of monocytes are also large, but have a kidney shape. The
cytoplasm of monocytes is greater in quantity compared to lymphocytes, and it
stains in a bluish-gray color. Monocytes have the capacity to leave the
bloodstream and enter tissue, where they mature into cells called
macrophages. Macrophages phagocytose (phagocytize) microbes and tissue debris.
There are three types of disorders that affect WBCs: leukocytosis, leukopenia, and
leukemia. Leukocytosis is a condition characterized by an abnormally high
number of mature WBCs. A common type of leukocytic illness is
mononucleosis, in which monocyte numbers greatly increase as a result of a
viral infection. Signs of this condition include enlarged spleen and lymph nodes.
Leukopenia is an abnormally low level of WBCs and commonly occurs with
the use of steroids such as cortisone. Like leukocytosis, leukemia is also
characterized by a high number of WBCs; however, this high number is a result of
cancerous proliferation of immature, nonfunctional WBCs.
Neutropenia is a condition in which neutrophils are abnormally low in the
body. Because these granulocytes play an important role in fighting infection,
individuals with neutropenia are more susceptible to infections. These infections are
also more likely to develop into life-threatening conditions. Neutropenia often occurs
in people undergoing chemotherapy or radiation therapy. Physicians may suspect
neutropenia in patients who frequently develop infections.
In order to test for neutropenia, a sample of blood is drawn. If the neutrophil count
falls below 500 cells per microliter, then the risk of infection increases, and the
ability of the body to fight infection decreases. There are various reasons why the
number of neutrophils decreases in the body. For instance, bacterial infections and
certain drugs can destroy neutrophils at a faster rate than they are produced by the
bone marrow. In the case of an autoimmune disease, people can produce antibodies that
target neutrophils, resulting in a neutrophil deficiency. Disorders in which the spleen
becomes enlarged may result in neutropenia because the spleen traps and destroys
neutrophils. There are also certain conditions that affect the bone marrow producing
neutrophils in the body, such as cancer, viral infections (influenza), bacterial
infections (tuberculosis), or vitamin deficiencies. Certain drugs and toxins can also
decrease the red bone marrows’ ability to produce neutrophils.
There are two types of neutropenia: acute and chronic. Neutropenia does not present any
specific symptoms; it is usually diagnosed when a patient has frequently occurring and
unusual infections. A physician will confirm his or her suspicions by ordering a
complete blood cell count to identify the problem. While neutropenia is expected in
patients undergoing chemotherapy or radiation therapy, if the etiology of neutropenia is
unclear, doctors will obtain a bone marrow sample using a needle. The bone marrow sample
is viewed under a microscope to determine the problem. Once the problem has been
diagnosed, treatment depends on the origin or cause (etiology) and severity. If the
problem is drug induced, then cessation of the drug is an option. Viral infections that
cause neutropenia (e.g., influenza) are a transient problem, and neutropenia will be
resolved once the infection has abated. When neutropenia occurs because of an underlying
disorder such as leukemia, the patient’s neutropenic condition may improve after cancer
treatments. However, a bone marrow transplant may be used to treat more serious forms of
cancer, aplastic anemia, and leukemia.
Platelets, as we described earlier are primarily involved in hemostasis. Platelets are smaller fragments of larger cells called
megakaryocytes. Megakaryocytes are large nucleated cells off of which
numerous platelets bud. Platelets, like red blood cells, also lack a nucleus. They do,
however, have a plasma membrane surrounding a cytoplasm and a few organelles. Platelets
are the smallest formed elements of blood with a diameter of only 1 to 2 micrometers.
Platelets are the cellular component in the process of hemostasis in which
a clot is ultimately formed to stop blood loss from a damaged blood vessel. Under normal
circumstances, platelets are repeled by the endothelial lining of blood vessels. This is
due to the endothelial cells releasing the prostaglandin prostacyclin. Upon vascular
injury prostacyclin levels fall, and, along with exposed collagen as a binding site,
platelets start adhering to the damaged area. Through a series of further steps,
platelets and other proteins will seal the damaged blood vessel wall by forming a blood
clot.
Figure 10
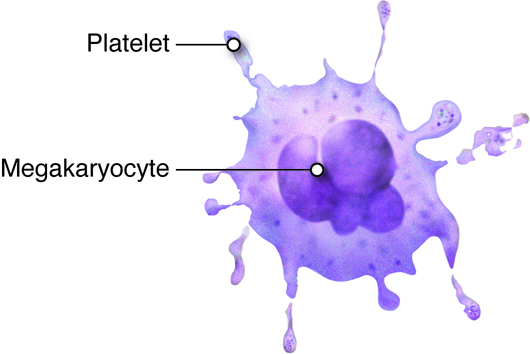
Hematopoiesis, or the formation of blood cells, occurs in the red bone
marrow of the body. Recall that seven different cell types (including platelets)
circulate in the blood, yet their lineage can all be traced to stem cells in the bone
marrow. As these stem cells produce new cells via mitosis, the daughter cells may follow
different maturational paths, depending on the needs of the body. Two common paths are
the myeloid cell line and the lymphoid cell line. The myeloid line gives rise to red
blood cells, granular leukocytes, platelets, and monocytes. The lymphoid cell line
produces T and B lymphocytes. Although most myeloid cells mature in the myeloid tissues
of the red bone marrow of the skull, vertebrae and rib cage (as well as long bones in
children), lymphoid derived cells typically mature in lymphoid tissues throughout the
body, such as the spleen, tonsils and lymph nodes.
Figure 11
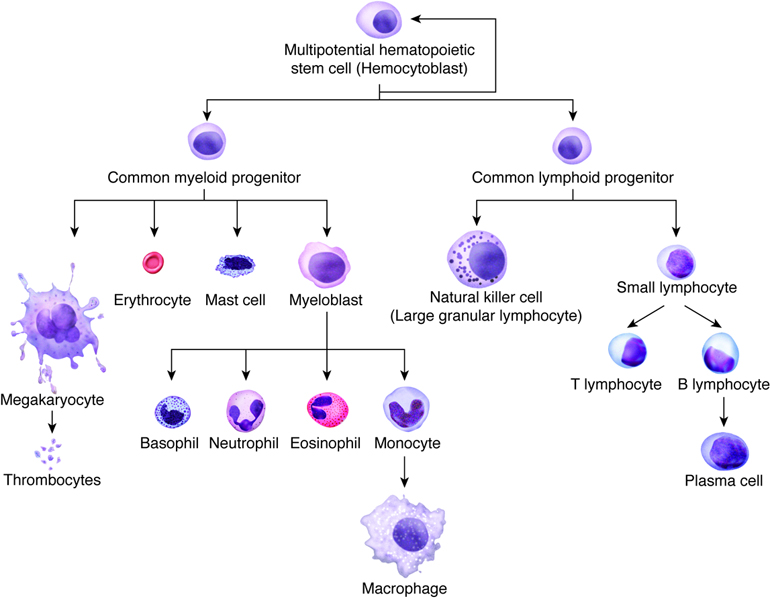
During embryonic development the formation of blood cells occurs in the liver, spleen,
and yolk sac. However, after birth, the infant’s liver and spleen become the locations
for destroying blood cells. All blood cells, whether they end up in the myeloid or
lymphoid family, begin as hemocytoblasts, stem cells that develop from embryonic
mesenchyme.
Formation of the Seven Cell Types in the Blood
| Progenitor Cell |
Formed Element |
Function |
| Proerythroblasts |
Red blood cells |
Transport oxygen and carbon dioxide |
| Myeloblasts |
Neutrophils |
Phagocytic |
|
Basophils |
Release heparin (anticoagulant) |
|
Eosinophils |
Phagocytic |
| Lymphoblasts |
Lymphocytes |
Provide specific immune response |
| Monoblasts |
Monocytes |
Phagocytic |
| Megakaryoblasts |
Platelets (thrombocytes) |
Hemostasis |
Within a blood vessel there are three main layers, which we will consider in vascular
tissue, described later. Endothelial cells are on the inside (lumen) of a blood vessel.
In some vessels, they are wrapped by smooth muscle cells. Fibroblasts secrete
extracellular matrix in the large vessels, creating a connective tissue outer
layer.
Figure 12
Endothelial Cells
Any surface that comes in contact with blood, including the inside of the heart
and every blood vessel, is lined with a special cell called an
endothelial (endo-inside) cell. Vascular
endothelium line the blood vessels, and there are special endothelial cells of
the lymph system. Endothelium of the interior surfaces of the heart chambers are
called endocardium.
The layer of endothelium is only one layer thick, and these cells have several
important functions.
Mechanical-chemical Stimulation
Endothelial cells sense changes in local chemical and mechanical environment, and
signal to the underlying muscle cells. For example, under certain conditions,
increased force on the vessels cause endothelial cells to release vasodilator
chemicals (such as nitric oxide) which cause the smooth muscle cells to relax,
leading to a dilated (expanded) vessel.
Immune Response
When tissues have been compromised by damage or invasion of pathogens, the immune
system releases chemical signals (cytokines) into the region. These cytokines
activate the endothelial cells to promote association with circulating immune
cells. Also, pores open up between endothelial cells to allow immune cells from
the circulation to leave the lumen of the vessel and invade the tissue.
Forming New Blood Vessels
As we grow or repair damaged tissue, we need new blood vessels to carry nutrients
and oxygen into new tissue. The process of blood vessel formation is called
angiogenesis, and involves stimulation of endothelial cells. Vascular
endothelial growth factor (VEGF) stimulates the normally quiescent
(non-dividing) endothelial cells to begin to divide. The cells then break down
the extracellular matrix and form new blood vessels. Without angiogenesis, we
could not repair any damaged tissue or adapt to changes. However, cancer cells
will often secrete VEGF or other angiogenic factors to cause the ingrowth of
vessels into the tumor, supporting its rapid growth.
Preventing Clot Formation
One important function of endothelial cells is only really observable as a
dysfunction: thrombus prevention. A thrombus, or a blood clot inside the vessel,
can occur because of the easily activated coagulation proteins that flow through
the blood stream.
Endothelial cell dysfunction, such as loss or inflammation, can cause clots to
form on the inner surface of the blood vessel. Large thrombi can occlude (or
completely block) blood vessels, so material cannot pass through. Just as
dangerous, these thrombi can dislodge from the vessel wall and may travel to the
vessels of the heart or brain and cause heart attacks or strokes,
respectively.
Smooth Muscle Cells
Smooth muscle tissue is found throughout the body, including around organs in the
digestive, respiratory, and reproductive tracts and around blood vessels. In the
cardiovascular system, smooth muscle cells form a layer outside of the
endothelial cells. Here they provide strength to blood vessels and provide a
mechanism for vessel adaptation.
Smooth muscle cells contract via the actin-myosin contraction mechanism described
in the muscular unit. Smooth muscle cells adjust their contraction based on
sensed stretch or the concentration of various chemical factors. For example,
hormones related to exercise or anxiety can cause vessel constriction and nitric
oxide can cause vasodilation.
Extra-Cellular Matrix of the Vessels
Besides the endothelial lining found in all blood vessels, and the smooth muscle
cell layer found in most vessels, all vessels except capillaries also have one
or more layers of connective tissue associated with them. These vessels have an
outer connective tissue layer (tunica externa, or adventitia), while some also
have a thin layer of connective tissue, called the internal elastic lamina that
is found between the endothelial layer and smooth muscle layer. The types and
concentrations of molecules in these connective tissue layers provide structure,
support, and determine how easily the vessels will passively (without relaxation
of muscle cells) expand.
Elastin
Even without active changes in smooth muscle tension, blood vessels need an
ability to deform and return to form when internal or external pressures are
applied. The elastin molecule in the connective tissue layers of blood vessels
gives them this property. Elastin, when combined with other
extracellular components, helps blood vessels and other tissues, including skin,
to return to their original shapes after expansion or deformation. Elastin
molecules are disorganized, entangled and crosslinked. When stretched, these
fibers straighten out, but will recoil back to normal when force is released on
the tissues.
Collagen
Collagen is a stiff, filamentous extracellular protein found in most
connective tissues. Collagen filaments are composed of twisted protofilaments,
and protofilaments themselves are also twisted. Thus, collagen has ropelike
qualities that provide stiffness. In blood vessels, collagen is found in the
tunicia externa and provides stiffness to the blood vessel to prevent rupture.
Arteries contain blood at higher pressure than veins so they have a thicker
layer of collagen than veins.
Blood as a Connective Tissue
Blood is a liquid connective tissue. Connective tissues are defined
as tissues with multiple cell types and a large extracellular matrix component.
The blood cells, described before, serve different functions and the plasma acts
as the extracellular matrix. Blood is a unique, complex fluid with various
physical characteristics and functions needed to maintain a stable internal
environment in the body. Blood volume is the combination of
hematocrit (45 percent) and plasma volume (55 percent).
Hematocrit (hct) is the fraction of blood that is composed of
red blood cells (RBCs). Besides RBC, leukocytes (white cells) and platelets make
up the rest of the formed elements. The process of producing blood cells is
called hematopoiesis. Stem cells are nascent cells in
the bone marrow that differentiate into various types of formed elements in
blood.
Conducting Heart Cells
There are three types of cells that comprise the myocardium: the pacemaker, the
conducting, and the myocardial cells. The pacemaker cells, also
called the nodal cells, are the cells found within the sinoatrial (SA) and the
atrioventricular (AV) nodes. These cells spontaneously depolarize, creating the
action potentials (or electrical impulses) that start the signal for atrial and
ventricular contraction. Specialized conducting cells carry the
action potentials at a high rate from the SA to the AV node, and then from the
AV node to the rest of the ventricular cells. Some of these specialized
conducting cells have been named after the people who described them. For
example, once action potentials move through the septum in the proximal part of
the AV node, they enter the Bundle of His and then move rapidly
through the Purkinje fibers that terminate in the ventricles.
Conducting cells are necessary to coordinate contraction by disseminating the
electrical signal through the ventricles.
Pacemaker Depolarization
The pacemaker cells in the nodes have the ability to spontaneously depolarize
until they reach their threshold value. In this way, they can produce action
potentials without the aid of nerves, neurotransmitters or neighboring cells.
Each of the action potentials they generate spreads through the heart and
results in a contraction. Because the SA node spontaneously depolarizes at a
faster rate than the AV node it typically paces the heart, a process called
overdrive suppression.
These pacemaker action potentials have significant differences from the action
potentials produced by skeletal muscle cells. Skeletal muscle cells have a
baseline or resting membrane potential that remains relatively constant and
highly negative (typically about -90 mV). The more negative potential of
skeletal muscle cells is due to their sustained permeability to potassium in the
resting state. Pacemaker cells have a much more positive resting potential (-55
mV), as they are a little less permeable to potassium in their resting state.
Their resting potential also slowly changes as the cells depolarize toward
threshold. This spontaneous depolarization, which occurs in the prepotential
period is due to the slow closure of their potassium channels (K+ channels) and
the slow opening of T-type calcium channels (Ca++ channels).
Remember that K+ channels allow for the efflux of potassium out of the cell,
which increases the negative charges along the inside of the cell membrane,
opposing depolarization. As these channels close, fewer positively charged ions
are leaving the cell. At the same time, other positive ions are entering the
cell (such as Calcium), allowing the cell to slowly become more positive.
The calcium channels allow for the influx of calcium from the extracellular
space. As these positive ions accumulate, it contributes to the rising membrane
potential. This sets the stage for threshold potential to be reached and an
action potential to fire.
When the threshold voltage is achieved, the action potential is initiated. In
skeletal muscle cells and neurons, the rapid depolarization at the beginning of
the action potential is due to the opening of voltage-gated sodium channels;
however, in cardiac pacemaker cells, these channels do not open. Instead,
L-type calcium channels open, allowing calcium to rapidly flow
into the cells to generate the upstroke. This dependence on external calcium to
generate an action potential instead of sodium is an important distinction
between cardiac and skeletal or neuronal cells.
Repolarization
After the rapid depolarization, the L-type calcium channels start closing, which
begins the process of repolarization, in which the membrane
potential becomes negative again. Along with the closing calcium channels,
potassium channels also open during the phase of repolarization. Cardiac
pacemaker cells do not hyperpolarize as do skeletal muscle cells or neurons. The
cycle is then repeated in which the potassium channels slowly close and the
calcium channels slowly open until an action potential is initiated. And so the
cycle goes; prepotential period, action potential, and repeat for our entire
lives.
Myocardial Cells
The third type of cell in the heart is the myocardial cell.
Comprising 99% of the mass of the heart, these cells are the cardiac
muscle cells that contract and carry out the flow generating function
of the heart. Without these cells, there would be no contraction.
Skeletal and Cardiac Muscle Similarities
Heart muscle, or cardiac muscle, is similar to the skeletal muscle you already
learned about. Both types of muscle contain sarcomeres and T tubules and depend
on calcium for contraction. Both muscles produce action potentials and contract.
Both contain mitochondria and require oxygen.
While similarities are evident between skeletal muscle cells and cardiac cells,
several important differences exist as well. These differences are what make the
heart a uniquely functioning organ. Cardiac muscle generally has a higher
concentration of mitochondria, which increases its requirement for oxygen. One
large advantage for having such a high concentration of mitochondria per cell is
that it does not fatigue. For a heart muscle that beats an average of 70 beats
per minute during every minute of every day, the need for energy is essential.
The disadvantage of a high mitochondrial content is that the amount of oxygen
required is quite high, which puts these cells at risk if blood flow to them is
ever limited, which is what occurs during heart attacks.
Skeletal and Cardiac Muscle Differences
These cells also differ in how they increase their cytosolic calcium for
contraction. Cardiac cells employ two sources of calcium for contraction. During
a cardiac action potential L-type calcium channels open, allowing calcium to
enter from the extracellular space. The entry of this calcium then stimulates
the release of calcium from internal calcium stores located in the sacroplasmic
reticulum. This is called calcium-induced calcium release. The
calcium then binds to the troponin, just like in skeletal muscle cells to
initiate contraction. In contrast, the cytosolic calcium in skeletal muscles
comes only from its intracellular stores and is released after an action
potential passes into the T-tubules.
There are other important differences between cardiac and
skeletal muscle. The
fibers of the cardiac muscle are short, while skeletal muscle cells
are long.
Cardiac cells are branched, while skeletal muscle cells are stacked
side by
side. Cardiac muscle cells contain only one or two nuclei, whereas
skeletal
muscle cells are multi-nucleated. Most importantly, cardiac muscle
cells are interconnected, while skeletal muscle cells are not.
Cardiac muscle cells are connected together by intercalated discs.
These disks physically connect adjacent cells, and include a set of specialized
proteins called gap junctions, that can directly pass electrical signals.
Because of this, depolarization of one cell can ultimately cause depolarization
of all the cells within the heart. This means that cardiac cells act together as
a single unit, allowing the heart to function as a whole to contract properly
and pump the blood efficiently. In the heart, gap junctions allow ions such as
calcium to flow quickly and unimpeded from one cardiac cell to the next The
presence of intercalated disks and gap junctions increase the electrical
conductivity of the heart, allowing the action potential to rapidly spread
throughout all the cells of the heart. If these differences between cardiac and
skeletal muscle cells did not exist, then the heart would probably not be able
to pump as efficiently.
The structural variations between the five major vessel types are due to the relative
thickness and composition of three vessel wall layers called tunics. The
specific function of each vessel type is dependent on the presence or absence of the
three tunics, as well as the extent of the tunic structures. The three tunics, from
inside to outside, are the tunica interna, tunica media, and
tunica externa.
Tunica Interna
The tunica interna forms the interior lining of blood vessels and is in direct
contact with the blood. This layer consists of endothelium, which
forms a continuous sheet of cells that lines the lumen of vessels. The
endothelial cells are involved in many vessel-related activities. The
endothelium is physically supported by the underlying basement membrane. The
basement membrane is composed largely of collagen fibers that
provide both strength and flexibility. The basement membrane anchors the
endothelium to the outermost component of the tunica interna, the internal elastic lamina. The internal elastic lamina is a sheet of
elastic fibers with numerous openings that allow movement of solutes between the
layers. The internal elastic lamina adheres the tunica interna to the tunica
media.
Tunica Media
The tunica media shows the greatest variation among the five vessel types. This
layer is composed of smooth muscle and connective tissue layers. In most vessel
types this is the thickest tunic. The smooth muscle cells of the tunica media
are oriented such that they encircle the lumen of the vessel. These
cells regulate the size of the vessel lumen by contracting or relaxing upon
stimulus of associated neurons, circulating hormones or local signaling
molecules. The flow and distribution of blood, as well as blood pressure, are
controlled by the dilation and contraction of blood vessels. The connective
tissue of the tunica media is composed of elastic fibers that are interspersed
within the muscle cell layers and that also form a separate sheet-like layer,
the external elastic lamina, between the tunica media and tunica externa. The
elastic fibers allow vessels to expand and recoil without loss of tension.
Tunica Externa
This tunic forms the outermost layer, merging with the surrounding tissues and
anchoring the blood vessel in place. The tunica externa is composed of mostly
collagen fibers. There are many nerves in the tunica externa and, in larger
vessels, a system of vessels that supply the vessel tissues called the
vasa vasorum.
Figure 12
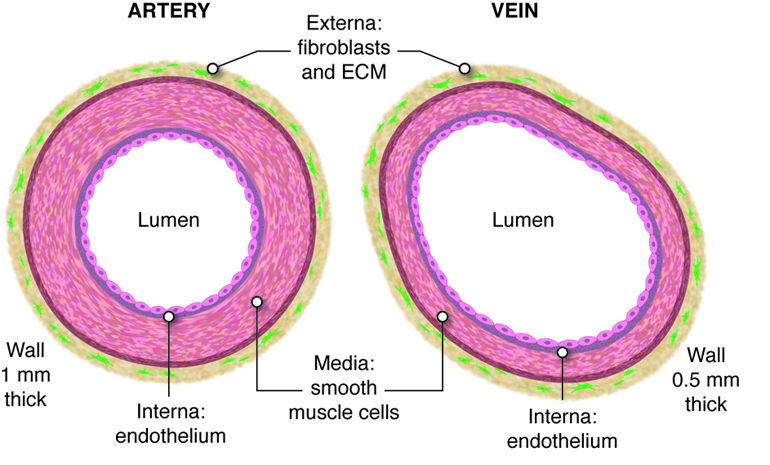
The heart resides just left of the midline of the body in the chest
cavity (thorax), and
is protected by the rib cage. It is made of two pumps that are
connected by a series of tube-like blood vessels that create the
circulatory system; the system of arteries, capillaries, and veins that
transport the blood to and from the heart. These
pumps are separated by a thick wall called the septum. The circulatory
system can be divided into two parts, the pulmonary circulation, which connects the
heart to the lungs; and the systemic circulation, which connects the heart
to the rest of the body.
The Heart
The heart is made of two pumps, each containing an atrium and a ventricle that is
separated by a thick wall called a septum. The heart pumps blood in one
direction through the circulatory system to provide oxygen to organs and to
remove metabolic waste from the tissues. Each heart beat forces blood through
the circulatory system and back to the heart. The blood enters the right atrium
and passes into the right ventricle. It is then ejected from the right ventricle
into the pulmonary artery, which carries blood from the heart to the lungs. Gas
exchange, which involves releasing carbon dioxide and acquiring oxygen, occurs
within capillaries of the lungs. Freshly re-oxygenated blood returns through the
pulmonary vein back to the left atrium. The blood passes from the left atrium
into the left ventricle. From there, it is ejected into the aorta and then into
systemic circulation.
In many ways, the heart is the center of the circulatory system. It is the pump that
controls the movement of blood. The arteries carry blood away from the heart; veins
carry it back to the heart. Blood circulates around the body, providing nutrients to
organs, while removing metabolic waste. What route does blood take? Blood enters the
right side of the heart by streaming into the right atrium, the chamber of
the heart that receives blood from the superior vena cava, inferior vena cava, and the
coronary sinus.
From the right atrium, it flows into the right ventricle. Blood then exits
the heart and enters pulmonary circulation, traveling through the
pulmonary artery (artery that carries blood from the heart to the
lungs) to the lungs where gases are exchanged. Freshly re-oxygenated blood returns to
the left atrium traveling through the pulmonary vein (vein
that carries blood from the lungs to the heart). From the left atrium, it flows into the
left ventricle before exiting the heart into the aorta to
enter the systemic circulation to be transported throughout the body. This oxygen-rich
blood flows through the arteries to the organs where oxygen is delivered and metabolic
waste is removed. From the organs, oxygen-poor (more specifically, less oxygenated)
blood returns to the heart, carried by the veins, and enters the right atrium again.
Figure 13
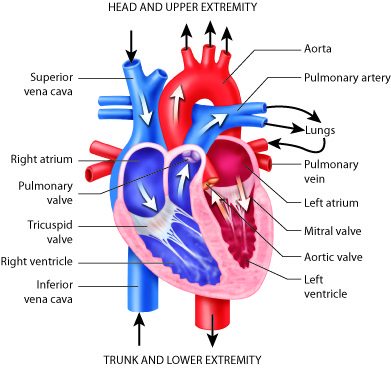
While these muscular pumps work together in the heart, they are not equal in the size of
their musculature. The left side contains thicker muscles than the right side. Why? The
right side has to generate less pressure in order to pump the blood through the short
circulatory pathway of the lungs. This does not require as much pressure as pumping to
the systemic circulation, like the left ventricle has to do.
Diseases of the Heart: Atrial Septal Defects
Have you ever heard of having a hole in the heart? A small percentage of people
have just that, a hole in the wall (septum) that separates the right and left
atria. Medically, this is known as an atrial septal defect. This
defect is a congenital disease, meaning it is present at birth. When a fetus is
still developing, the septum between the right and left atrium is not fully
formed. The atria are connected together by a hole, called the foramen ovale,
between the two chambers. This opening allows blood in the fetal heart to be
shunted from the right atrium into the left atrium. This shunted blood does not
get pumped into the pulmonary artery, as this is not necessary in the fetus.
Instead, the fetus receives oxygen (and nutrients) from the placenta via the
umbilical cord. When delivery occurs and the newborn takes his or her first
breath, the lungs inflate. This changes the pressure distribution in the heart
and causes a flap of tissue to close the foramen ovale, which then grows into a
completed septum. When the foramen ovale does not close properly, or if there
are similar congenital defects, the child is left with an atrial septal defect
or, in other words, a hole in his or her heart.
Whether or not this problem needs to be corrected is mainly dependent on the size
of the hole. This is because some blood from the left atrium will move through
the atrial septal defect into the right atrium, causing it to recirculate
through the pulmonarycirculation. If too much blood is shunted back through the
lungs it can cause pulmonary hypertension and ultimately heart failure.
Most of the time, symptoms are very mild or not even noticed. Sometimes, symptoms
do not develop until much later in life. Atrial septal defects can be treated
with surgical closure of the defect.
As mentioned earlier, blood flows through the heart in one direction. What prevents blood
from flowing in the opposite direction? The answer is the heart’s valves. These valves
maintain unidirectional flow by preventing backward (retrograde) flow when the pressure
gradients change during the heart cycle. These valves are found in matched pairs in two
locations of the heart. The first set, the atrioventricular (AV) valves,
separate the atria from the ventricles. The second, the semilunar valves,
are located between the ventricles and the arteries they feed.
Following the path of blood flow through heart, the first valve is the tricuspid
valve found separating the right atrium from the right ventricle. This AV
valve is so named because it has three flaps: the anterior cusp, the
medial cusp, and the posterior cusp. On the ventricle side
of the valve are long muscular cords, called chordate tendinae, that extend from the
inferior side of each cusp. The chordate tendinae attach to each cusp on one end and to the
papillary muscle, which is connected to
the inner wall of the
ventricle, on the other end. These muscles prevent the valves from
inverting back into the atria during ventricular contraction.
Figure 14
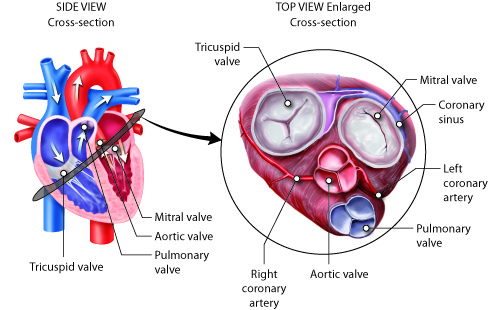
Most of the blood flows passively into the atria, through the tricuspid valve, and into
the right ventricle. Atrial contractions then push most of the remaining atrial blood
into the ventricle. As the right ventricle fills, the pressure inside it increases. This
increased pressure, coupled with the right atria moving into its relaxation phase,
causes the ventricular pressures to be greater than atrial pressures. This causes the
tricuspid AV valves to close. In this closed position, the chordae tendinae that are
attached to the ventricular side of the valves are fully extended and taut, like a
stretched bungee cord. The chordae tendinae prevent the higher pressure in the ventricle
from forcing the valve to open back into the atria, thus preventing the backflow of
blood through the tricuspid valve.
As the blood flows from the right ventricle into the pulmonary artery, it passes through
the pulmonary semilunar valve (pulmonary valve). Opposite to the tricuspid, the pulmonary
semilunar valve remains closed when the ventricle is filling and opens during
ventricular contraction.Again, this is because the valves work by opening when the
pressure is higher on their proximal side and close when the pressure is higher on their
distal side.
On its return from the lungs, blood enters the left atrium. As the blood passes from the
left atrium into the left ventricle, it passes through the bicuspid valve,
often referred to as the mitral valve. This valve is similar in structure
and function to the tricuspid, but only contains two cusps, the anterior and posterior
cusps. It, too, is open when the pressure in the atria exceeds the ventricle and it,
too, has chordae tendinae and papillary muscles that prevent its inversion upon
ventricular contraction. The release of blood from the left ventricle into the aorta is
regulated by the aortic semilunar valve, which is most commonly referred to
as the aortic valve. It is similar in structure and function to the
pulmonary semilunar valve. Because they regulate the flow of blood through the heart and
prevent its backflow, these valves are indispensable for the proper function of the
heart.
Diseases of the Heart: Ruptured Chordae Tendinae
The chordae tendinae perform an important function by keeping the cusps of the
atrioventricular valves tethered to the interior wall of the ventricles. Without
these tendons, and their associated papillary muscles, the valves could become inverted when the pressure in the
ventricle increases, causing the blood to rush back into the atria.
Although this happens infrequently, the chordae tendinae can rip or rupture. When
this happens, the cusps of the tricuspid or mitral valves are no longer properly
tethered to the wall of the ventricle. As the blood presses back
against the valves during ventricular contraction, the valves may not remain
closed. If many of these connections are ruptured or if the thicker ones are
affected, the valves can open in the reverse direction, and blood can backflow
into the atria. This results in increased pressure in the atria and, if the
backflow is significant enough, congestive heart failure could develop.
Rupture of the chordae tendinae occurs when a patient suffers from a myocardial
infarction (heart attack), heart valve infection, or from trauma to the chest.
Damage can range from minor to major depending on the number of chordae
affected. It is diagnosed by hearing a murmur, or a characteristic whooshing
noise, upon auscultation (listening to heart sounds with a stethoscope or other
device) of the heart. Ruptured chordate tendinae, and their associated
conditions, commonly lead to heart failure.
Rupture of the chordae tendinae attached to the mitral valve is most common,
although rupture of those bound to the tricuspid valve can also occur. Rupture
of the mitral valve chordae tendinae can result in a rapid increase in left
atrial pressure. This can subsequently increase the pressure in the pulmonary
circulation, ultimately producing pulmonary edema; which is retention of fluid
in the lungs. An echocardiogram can provide a more detailed look into the condition
of the heart and the degree of damage to the chordae tendinae.
Blood circulates in a closed system. It flows from the heart, through the lungs, back to
the heart, and then to the rest of the body. The blood always returns to the heart. The
amount of blood that circulates and the rate blood circulates is controlled by the
pumping of the heart. The system is similar to having a rubber ball filled with water
and a tube connected on both sides of it. When the ball is squeezed, the water enters
the tube on one side. When the ball is relaxed, water is drawn into it through the other
side. Squeeze again, and more water is ejected into the tube. Relax again, and water is
drawn into the ball. The valves between the opening of the tube and the ball, which only
open one way, control the entry of water into the tube and prevent its tendency to move
backwards.
Blood continuously flows in a circle. Oxygen-poor blood enters the lungs where carbon
dioxide, a waste product of the organs’ cells, is exchanged for oxygen. This oxygen-rich
blood returns to the heart by way of the pulmonary veins. When it arrives from the
lungs, it enters the left atrium. Some of the blood flows passively through the bicuspid
(or mitral) valve into the left ventricle. When the left atrium contracts, the remaining
blood in the left atrium is pushed through the bicuspid valve into the powerful left
ventricle.
After the left ventricle contracts, the blood exits through the aortic valve into the
aorta to be circulated throughout the body. The first section of the aorta is the
ascending aorta, which runs only a short way before becoming the aortic arch at the apex
of an inverted U, from which vessels branch to supply the head, neck and arms. After the
aortic arch turns downward it becomes the descending aorta. The thoracic aorta supplies
blood to the thorax above the diaphragm and the abdominal aorta supplies blood to the
region below the diaphragm. Arteries that branch off of the aorta continue to branch
into smaller and smaller arterioles and eventually form the capillaries within the
organ. It is within the capillaries that the gas exchange occurs. In the organs, oxygen
is provided and carbon dioxide is removed. This is the opposite of gas exchange in the
lungs where carbon dioxide is removed and oxygen is provided.
Once exchange has occurred, oxygen-poor blood exits the capillaries, flowing into
venules, or small veins. These venules combine to form veins, which eventually return
the blood to the right atrium. The blood from the head, neck and arms returns to the
right atrium through the superior vena cava, while the blood from the lower
body returns through the inferior vena cava. The right atrium contracts and
blood passes through the tricuspid valve into the right ventricle. From the right
ventricle, the blood is ejected through the pulmonary semilunar valve into the pulmonary
artery to travel to the lung arterioles and capillaries. Oxygen-rich blood flows into
the venules and back to the heart through the pulmonary vein and is ready to enter the
left atrium again and circulate around the body again.
Figure 15
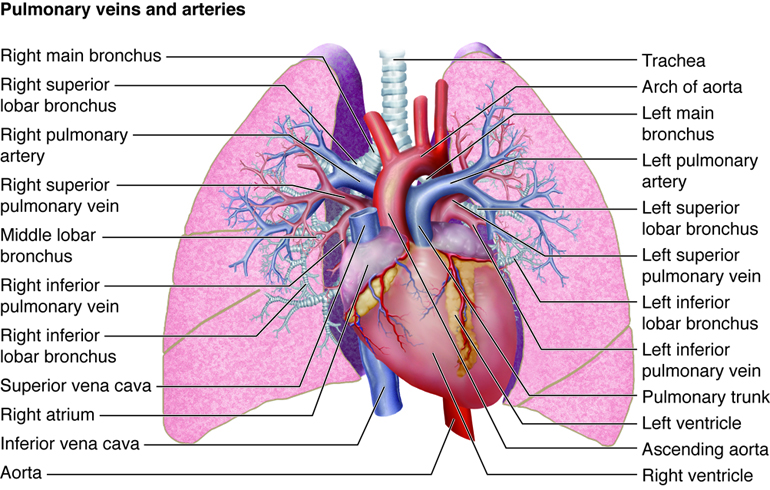
The Fetal Circulation
The path that blood takes through the fetal circulatory system and heart differs
somewhat from that in the postpartum state. In particular, a majority of the
blood passes through three shunts, bypassing the liver and lungs that do not
require as much blood in the fetus as they do after birth. These shunts include
the ductus venosus, which bypasses the liver, and two shunts that bypass the
lungs; the foramen ovale and the ductus arteriosus.
As highly oxygenated blood travels along the placental vein into the fetus, some
of the blood perfuses the liver, while a majority bypasses the liver through the
ductus venosus and directly enters the inferior vena cava. The fetal liver
matures late in development, when it prepares to take over functions such as
processing chemicals and nutrients absorbed by the GI tract. Because it is not
carrying out such functions in the fetus, there is not a need to send
significant blood flow there.
The lungs are also not functional in the fetus, as the placenta carries out the
functions of gas exchange. In fact, the lungs are deflated, with the alveoli
collapsed. This condition also narrows the vessels of the pulmonary vasculature,
creating high resistance to blood flow. To keep the workload on the right heart
from becoming too high in the fetus, most of the blood bypasses the lungs. This
occurs via two shunts. The first is an opening between the right and left atria
called the foramen ovale. Because the pressures are higher in the right heart
than the left heart in the fetus (due to the high pulmonary resistance), blood
moves from the right atrium to the left atrium through this shunt. The remaining
blood moves into the right ventricle where it is pumped into the pulmonary
artery. But even from here most blood will not go through the remainder of the
pulmonary circulation. Instead, it travels from the pulmonary artery to the
aorta through the ductus arteriosus.
Soon after birth the lungs inflate, greatly reducing the resistance through
pulmonary circulation and lowering the pressures on the right side of the heart.
This briefly reverses flow through the foramen ovale, pushing two flaps of
tissue over the opening, closing it. These flaps grow into the atrial septum,
leaving a slight depression called the fossa ovalis. The decreased pressure in
the pulmonary artery also triggers the ductus arteriosus to collapse, converting
it into the connective ligamentum arteriosum over the next three months or so.
The ductus venosus become a connective tissue remnant, called the ligamentum
venosum, found on the inferior surface of the liver.
Aging and the Heart: Valvular Stenosis
If any of the four valves within the heart become stiff, the valves are unlikely
to open to the fullest extent. Blood flow is impeded and the pressure in at
least one of the heart chambers increases.
Mitral valve stenosis increases the stiffness of the mitral valve that separates
the left atrium from the left ventricle. This results in increased pressure in
the left atrium, which backs up into the pulmonary circulation, causing edema
there. If the atria can’t compensate enough to maintain flow through the
narrowed mitral valve, heart failure will develop. People with untreated mitral
stenosis typically develop atrial hypertrophy in an effort to generate enough
atrial pressure to maintain this flow. Similar results and compensatory
mechanisms occur in tricuspid valve stenosis.
Aortic valve stenosis occurs when the aorta valve stiffens. This increases the
pressure in the left ventricle and increases the stress developed in the wall of
the left ventricle during ejection. The stroke volume is reduced as the left
ventricle has to contract more forcefully to eject the blood into the aorta.
This causes left ventricular hypertrophy, although this is not always enough to
maintain flow.
The heart is protected by the ribcage in the chest cavity. It is enclosed in a sack that
provides increased protection. This sack, or pericardium,
is a
fluid-filled, (although the space is very small, so the volume of
fluid is very low) membranous structure that separates the heart from
the lungs within the
chest. It is made up of the visceral pericardium,
which is actually the outer epicardial layer of the heart, and the
parietal pericardium.
At times, the pericardium can become inflamed, which is a condition
called
pericarditis.
Figure 16
The heart is made of three layers, the endocardium, the myocardium, and the epicardium.
The endocardium is the innermost layer of the heart that forms a barrier
between the muscle layers of the heart and blood. The myocardium is the
thickest of the three layers of heart tissue. It is the cardiac muscle layer that
contracts and generates force to pump the blood. The outermost layer of the heart is the
epicardium. It is a thin layer of cells that covers the outside of the
heart and forms the visceral layer of the pericardium. The epicardium protects the heart
within the thorax.
Diseases of the Heart: Cardiac Tamponade
Inflammation of the pericardium can result in cardiac tamponade.
When the pericardium gets infected fluids or blood can accumulate between the
layers of the pericardium. This increases the pressure that is exerted on the
outside of the heart and prevents the heart from filling appropriately during
diastole. If the heart does not fill normally, the stroke volume decreases. The
body can try to compensate with an increased heart rate and force of contraction
(both effects of the sympathetic nervous system), but if this is not enough, the
person will go into heart failure.
Force generating cells of the heart require energy to beat constantly and the conductive
cells require energy to maintain electrical potentials. So the heart, itself, requires a
large supply of blood. It is the role of the coronary circulation to
deliver this blood to the heart. In this circulation, the coronary arteries deliver the
oxygen-rich blood to the myocardium while the cardiac veins are responsible for
returning the blood back to the right atrium.
Coronary arteries on the surface of the heart are called epicardial coronary arteries.
These arteries are commonly affected by atherosclerosis and can become blocked, causing
angina or heart attack. The branches of the coronary arteries penetrate the myocardium
and work their way inward, ending up as subendocardial arteries.
The right and left coronary arteries originate from the aortic sinuses,
which exit from the aorta right after it leaves it heart. The posterior
interventricular branch of the right coronary artery supplies the right and
left ventricles. The right ventricle also receives blood from the second branch of the
right coronary artery, the marginal branch. The left coronary artery has
two branches: the anterior interventricular branch (left anterior
descending branch), which supplies both ventricles, and the circumflex
branch, which supplies the left ventricle and left atrium.
The human cardiovascular system consists of two separate circulatory routes, the
pulmonary circuit and the systemic circuit. In each
circuit, several levels of blood vessels work in sequence to receive blood from the
heart, deliver it to the appropriate tissues and cells, and then return blood to the
heart. In general, the arteries and arterioles of the systemic
circuit deliver blood, rich with oxygen and nutrients, to capillary
networks interspersed within the organs and tissues. The systemic venules
and veins drain the capillary networks and return the blood, high in carbon
dioxide and waste products, to the right side of the heart. In contrast, the arteries
and arterioles of the pulmonary circuit deliver oxygen-poor blood to the capillaries
surrounding the alveoli (singular: alveolus) of the lungs. After carbon dioxide is
exchanged for oxygen, the pulmonary venules and veins return oxygen-rich blood to the
left side of the heart. The arteries, arterioles, capillaries, venules, and veins are
categorized by wall structure, location in the circuit, and function.
Figure 18
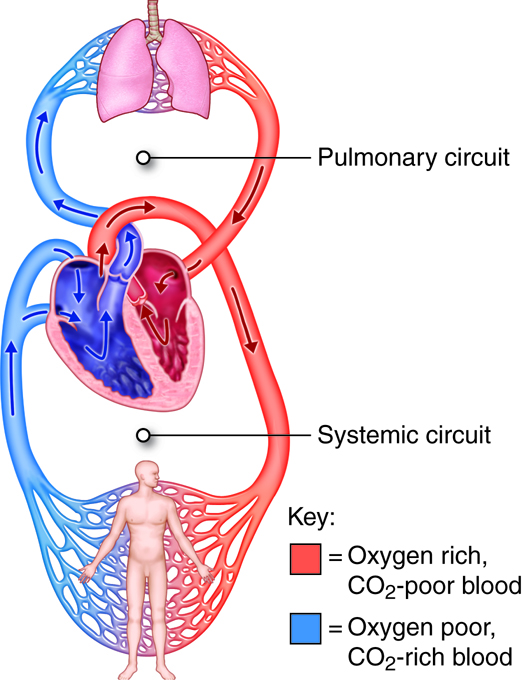
Blood from the left ventricle is pumped into the aorta. This is the beginning of the
systemic circulation. The aorta and its branches deliver blood to all body tissues
except for those in the lungs. Venous blood from the systemic circuit is returned to the
right atrium by way of the venae cavae and the coronary sinus. The superior vena cava
receives blood from veins that drain the head, neck, chest, and upper limbs. The
inferior vena cava receives blood from veins that drain the abdomen, pelvis, and lower
limbs. The coronary sinus drains the myocardium.
Figure 19
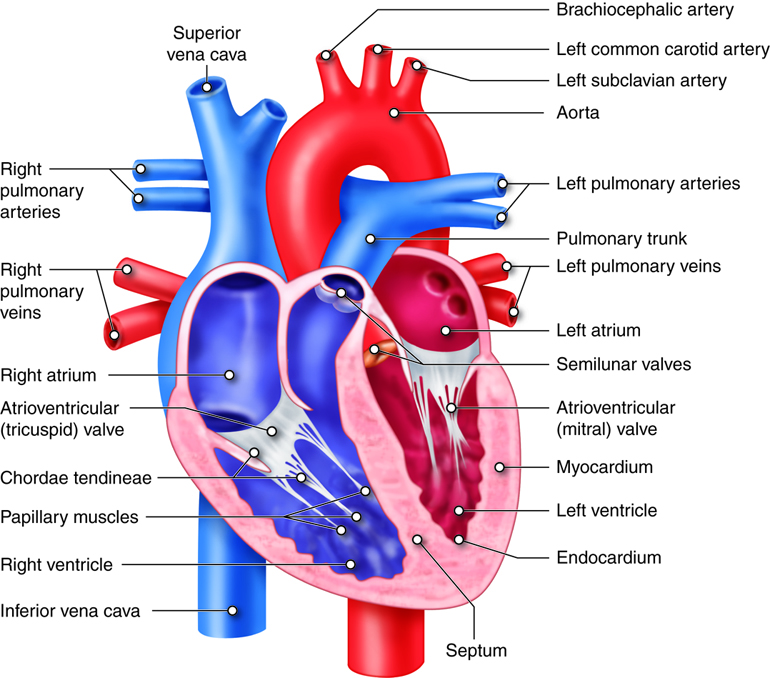
The aorta is divided into the ascending aorta, the arch of the aorta, the thoracic aorta,
and the abdominal aorta. Branches off of the ascending aorta supply the atria and ventricles of
the heart. The aortic arch has three major branches: the brachiocephalic trunk, left
common carotid artery, and left subclavian artery. These arteries and their branches
supply the head, neck, brain, and upper limbs. Branches of the thoracic aorta and
abdominal aorta supply structures of the thorax and abdomen, respectively. Branches of
the abdominal aorta also supply the lower limbs.
Figure 20
The arterial supply of the brain includes an arrangement of arteries called the arterial
circle (circle of Willis). These vessels provide collateral pathways that ensure
uninterrupted blood supply to the brain if some arteries are blocked. The venous
drainage of the brain is unique in that blood drains first into large chambers called
dural sinuses instead of veins.
Figure 21
The arterial supply of the thorax and abdomen includes both visceral and parietal
branches. The visceral branches perfuse the organs, and the parietal branches perfuse
the wall structures. Venous drainage of the digestive organs includes the hepatic portal
circulation, which sends blood to the liver for nutrient processing and toxin removal
before it is returned to the systemic circulation.
Figure 22
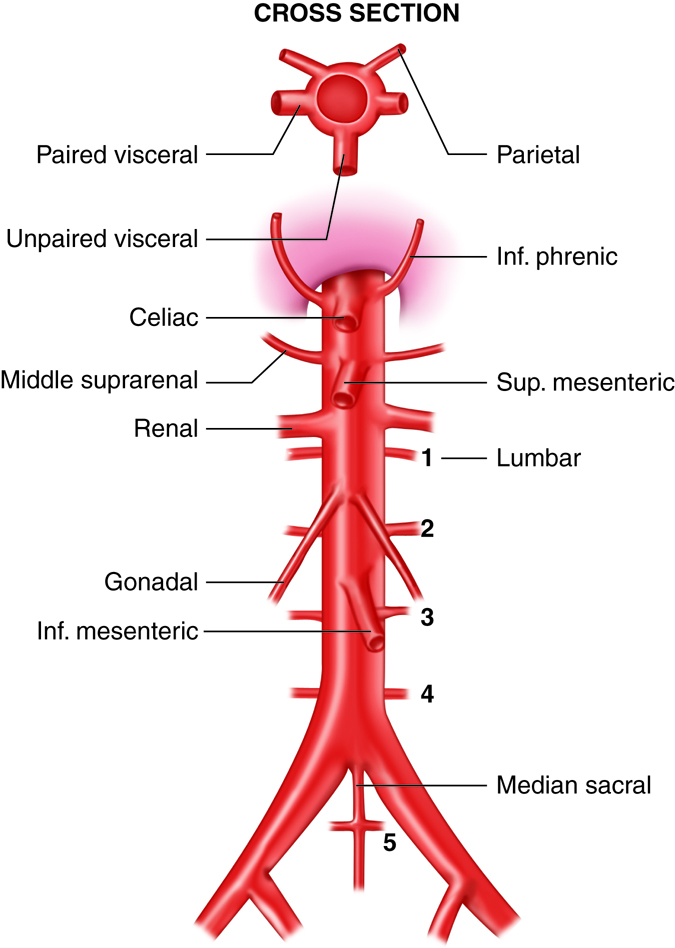
The arterial supply of the upper limbs is primarily a continuing sequence of vessels
rather than branches of other vessels. The subclavian artery continues as the axillary
artery, which continues as the brachial artery.
The pelvis and lower limbs are supplied by the internal and external iliac arteries,
respectively. These arteries are branches of the right and left common iliac arteries,
which are the terminal branches of the abdominal aorta.
Figure 23
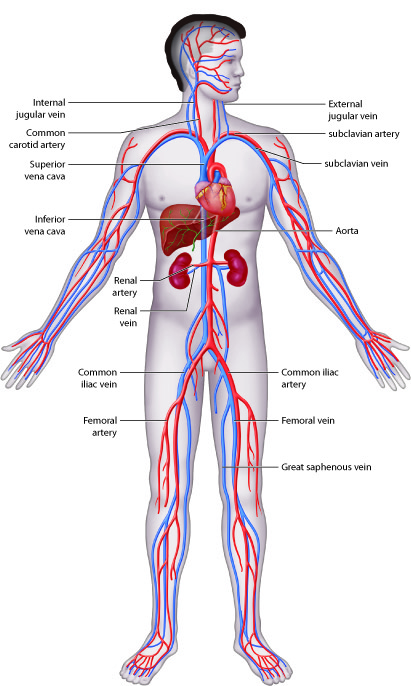
The aorta is the biggest artery in the body, with a diameter of
approximately 3 cm (1 in.). All systemic arteries branch off the aorta. The aorta has
four major segments: ascending aorta, arch of the aorta (or
aortic arch), thoracic aorta, and abdominal aorta. Arteries
that branch off each section of the aorta divide into smaller arteries that supply
different organs. These arteries then divide into arterioles within the organs, and
finally into capillaries that supply all systemic tissues except the alveoli of the
lungs.
The Ascending Aorta
The ascending aorta, which is approximately 5 cm (2 in.) long, is the first
section of the aorta. It begins at the aortic valve, at the upper part of the
base of the left ventricle. It has three dilations called aortic sinuses. The
right and left coronary arteries originate from the right and left
aortic sinuses. The posterior interventricular branch of the right
coronary artery supplies the right and left ventricles. The right ventricle also
receives blood from the second branch of the right coronary artery, the
marginal branch. The left coronary artery has two branches: the
anterior interventricular branch (left anterior
descending branch), which supplies both ventricles, and the
circumflex branch, which supplies the left ventricle and left
atrium.
The Arch of the Aorta
The ascending aorta curves to the left and becomes the arch of the aorta, which
is about 4–5 cm (2 in.) long. It runs downward and ends in front of the border
between the fourth and fifth thoracic vertebrae. The arch of the aorta gives
rise to three major branches: the brachiocephalic trunk, left common carotid
artery, and left subclavian artery.
Branches of the Arch of the Aorta
| Branch |
Area Supplied |
Description |
| Brachiocephalic trunk |
Right upper limb, head, neck |
Brachiocephalic trunk (brachiocephalic artery)
is the largest branch of the aortic arch; Gives rise to the right
subclavian artery and right common carotid
artery
|
| Right subclavian artery |
Right upper limb, brain, spinal cord, neck, shoulder, thoracic viscera
and wall, scapular muscles |
Runs from the brachiocephalic trunk and to the first rib before passing
into the armpit (axilla) |
| Internal thoracic artery |
Anterior thoracic wall, mediastinum structures |
The internal thoracic artery branches from the first part
of the subclavian artery; Ends at the sixth intercostal space; Used to
create the bypass for single coronary artery bypass grafting |
| Vertebral artery |
Posterior part of brain |
The right vertebral artery branches off from the right
subclavian artery and passes through the foramen magnum to reach the
inferior surface of the brain; Joins with the left vertebral artery to
form the basilar artery, branches of which supply the
cerebellum and pons of the brain, and the inner ear |
| Axillary artery |
Shoulder, thoracic and scapular muscles, humerus |
The axillary artery is the part of the subclavian artery
that passes into the armpit; The same vessel has different names as it
passes through different areas of the body |
| Brachial artery |
Upper limb |
The axillary artery becomes the brachial artery in the arm,
where it is easy measure BP; To control bleeding, the brachial artery
should be compressed near the middle of the arm |
| Radial artery |
Radial (lateral) aspect of forearm, wrist, hand |
The radial artery is a direct continuation of the brachial
artery very near the skin surface at the wrist, where the radial pulse
is typically measured |
| Ulnar artery |
Ulnar (medial) aspect of forearm, wrist, hand |
The ulnar artery is the larger of the two brachial artery
branches; Reconnects with the smaller branch (the radial artery), in the
palm, creating the superficial palmar arch and the
deep palmar arch
|
| Superficial palmar arch |
Branches supply palm and fingers |
Lies over the flexor tendons of the fingers and extends to the palm;
Gives rise to the common palmar digital arteries that
perfuse the palm, each of which divides into a pair of proper
palmar digital arteries that perfuse the fingers |
| Deep palmar arch |
Branches supply the palm and fingers |
Lies below the flexor tendons of the fingers and extends to the palm;
Gives rise to palmar metacarpal arteries that perfuse the
palm and join with the common palmar digital arteries |
| Right common carotid artery |
Head and neck (right side) |
Begins where the brachiocephalic trunk divides into its two branches;
Divides into the right external and right internal carotid arteries;
Often used to measure the pulse (at the side of the neck), for example,
when exercising or administering cardiopulmonary resuscitation |
| External carotid artery |
Face, scalp, neck |
Near the temporomandibular joint, the external carotid artery divides into the superficial temporal and maxillary arteries;
Used to detect the carotid pulse |
| Internal carotid artery |
Orbital structures (including the eyeball), ear, cerebrum, pituitary
gland, external nose |
Branches of the internal carotid artery: the
anterior cerebral arteries supply parts of the frontal
and parietal lobes; Branches merge with branches of the basilar
artery—the posterior cerebral arteries (that supply the
occipital lobes)—to form the cerebral arterial circle (circle of Willis) at the base of the brain;
Posterior communicating arteries connect the posterior
cerebral arteries with the internal carotid arteries; Anterior
communicating arteries connect the anterior cerebral
arteries; Cerebral arterial circle (which also includes the internal
carotid arteries) equalizes BP in the brain and keeps blood flowing to
the brain if other arteries are damaged |
| Left common carotid artery |
Head and neck (left side) |
The left common carotid artery branches from the arch of
the aorta and divides into branches with the same names as branches of
the right common carotid artery |
| Left subclavian artery |
Left upper limb |
The left subclavian artery branches from the arch of the
aorta; Its branches and their names are similar to those of the right
subclavian artery |
The Thoracic Aorta
As the aorta continues its descent, it passes through the aortic hiatus, an
opening in the diaphragm. The part of the aorta between the arch of the aorta
and the diaphragm is called the thoracic aorta. It is approximately 20 cm (8
in.) long and starts at the border between the fourth and fifth thoracic
vertebrae. The thoracic aorta gives rise to a number of small arteries. The
visceral branches supply the viscera, and the parietal
branches supply the body wall structures of the thorax.
Branches of the Thoracic Aorta: Visceral
| Branch |
Area Supplied |
Description |
| Pericardial arteries |
Pericardium |
Two or three of these tiny pericardial arteries perfuse
the pericardium |
| Bronchial arteries |
Pleurae, bronchial tubes, bronchial lymph nodes, esophagus |
Right bronchial artery branches from the third posterior
intercostal artery; Two left bronchial arteries branch from the thoracic aorta |
| Esophageal arteries |
Esophagus |
Four or five esophageal arteries perfuse the esophagus
|
| Mediastinal arteries |
Mediastinum structures |
Numerous small mediastinal arteries
|
Branches of the Thoracic Aorta: Parietal
| Branch |
Area Supplied |
Description |
| Posterior intercostal arteries |
Intercostal, pectoralis major and minor, and serratus anterior muscles;
overlying subcutaneous tissue and skin; mammary glands; vertebrae,
meninges, spinal cord |
Nine pairs of posterior intercostal arteries |
| Subcostal arteries |
Same as the posterior intercostal arteries |
The subcostal arteries derive their name from their
location below the rib cage (costal, rib area) |
| Superior phrenic arteries |
Superior and posterior aspects of diaphragm |
The small superior phrenic arteries arise from the lower
part of the thoracic aorta |
The Abdominal Aorta
The abdominal aorta is the part of the aorta between the diaphragm and its
bifurcation (division into two branches) into the two common
iliac arteries at the level of the fourth lumbar vertebra. The abdominal aorta
is about 13 cm (5.1 in.) long. Like the thoracic aorta, the abdominal aorta
gives off visceral and parietal branches.
Branches of the Abdominal Aorta: Unpaired Visceral Branches
| Branch |
Area Supplied |
Description |
| Celiac trunk |
|
After emerging from the abdominal aorta, the celiac trunk divides into the left gastric artery, splenic
artery, and common hepatic artery
|
| Left gastric artery |
Stomach, esophagus |
Smallest of the three branches of the celiac trunk |
| Splenic artery |
Branches supply pancreas, stomach, greater omentum |
Largest branch of the celiac trunk; Three branches: pancreatic
artery (supplies the pancreas), left gastroepiploic
artery (stomach and greater omentum), short gastric
artery (stomach) |
| Common hepatic artery |
Branches supply liver, gall bladder, stomach, duodenum, pancreas,
greater omentum |
Intermediate-sized branch of the celiac trunk; Three branches:
proper hepatic artery (supplies the liver, stomach, and
gall bladder), right gastric artery (stomach),
gastroduodenal artery (stomach, greater omentum,
duodenum, and pancreas) |
| Superior mesenteric artery |
Branches supply duodenum, pancreas, parts of small and large
intestines |
The superior mesenteric artery runs between the layers of
mesentery; Five branches: inferior pancreaticoduodenal artery (supplies the duodenum and pancreas), jejunal artery (jejunum of small intestine), ileal artery (ileum of
small intestine), ileocolic artery (ascending colon of
large intestine), middle colic artery (transverse colon of
large intestine) |
| Inferior mesenteric artery |
Branches supply parts of large intestine |
The inferior mesenteric artery has three branches:
left colic artery (supplies transverse and descending
colons of large intestine), sigmoid arteries (sigmoid and
descending colons of large intestine), superior rectal artery (rectum) |
Branches of the Abdominal Aorta: Paired Visceral Branches
| Branch |
Area Supplied |
Description |
| Suprarenal arteries |
Adrenal glands |
Only the middle pair of suprarenal arteries arises from
the abdominal aorta; Superior pair arises from the inferior phrenic
artery; Inferior pair arises from the renal arteries |
| Renal arteries |
Kidneys, adrenal glands, ureters |
Right renal artery is longer than the left |
| Gonadal arteries |
Ureters, ovaries, fallopian tubes (females); testes, epididymis
(males) |
In males, the gonadal arteries are called the
testicular arteries; In females, they are called the
ovarian arteries (which are much shorter than the
testicular arteries) |
Branches of the Abdominal Aorta: Unpaired Parietal Branch
| Branch |
Area Supplied |
Description |
| Median sacral artery |
Sacrum, coccyx |
The median sacral artery originates in the posterior part
of the abdominal aorta, about 1cm above the bifurcation into the right
and left common iliac arteries |
Branches of the Abdominal Aorta: Paired Parietal Branches
| Branch |
Area Supplied |
Description |
| Inferior phrenic arteries |
Diaphragm (inferior aspect), adrenal glands |
The inferior phrenic arteries are the first paired
branches of the abdominal aorta; Arise from just above the origin of the
celiac trunk and, occasionally, from the renal arteries |
| Lumbar arteries |
Lumbar vertebrae, spinal cord and it meninges, back muscles and skin in
the lumbar region |
Four pairs of lumbar arteries
|
Arteries are vessels that carry blood away from the heart. The tunica media is the
thickest tunic in arteries, although all three layers are present. The smooth muscle
cells in the tunica media of arteries are innervated by fibers of the autonomic nervous
system. When stimulated, the muscle cells contract and cause
vasoconstriction, a decrease in the diameter of the blood vessel lumen.
Relaxation of the muscle cells causes vasodilation, an increase in diameter
of the lumen. Damage to an artery also triggers vasoconstriction to help prevent blood
loss. As arteries move blood away from the heart, they branch into smaller and smaller
vessels.
Artery Types
Arteries are typically classified by location, but they can also be described by
their function.
Elastic Arteries
As the name indicates, elastic arteries have vast capacity for
expansion and retraction. These properties are due to the internal and external
elastic laminae and extensive elastic fiber sheets interspersed within the
muscle cells. The arteries with the largest diameters in the body are elastic
arteries. The elastic arteries that receive blood from the heart, such as the
aorta, the common carotids, and the pulmonary trunk, play a critical role in
regulating the pulsing blood that is ejected from the heart. These vessels
temporarily store blood by expanding and then slowly recoiling. This action
propels the blood forward at a more consistent rate than is delivered during
systole and also helps to maintain blood pressure during diastole. The elastic
arteries are also called conducting arteries because they direct
blood to the next smallest diameter vessels, the muscular arteries.
Muscular Arteries
Compared to elastic arteries, muscular arteries have a smaller
diameter and a higher percent of muscle cells to elastic fibers in the tunica
media. Muscular arteries are typically medium-sized vessels with a very thick
tunica media. The extensive smooth muscle cells in muscular arteries allow for
greater vasoconstriction and vasodilation. This property of muscular arteries
contributes to the regulation of blood flow. Muscular arteries are also called
distributing arteries because they direct blood into the
various organs, continuing to branch into smaller and smaller diameter vessels.
Some examples of muscular arteries are the brachial, radial, ulnar, and coronary
arteries.
The head and neck are supplied by the paired common carotid arteries and three paired
branches of the right and left subclavian arteries: the vertebral arteries,
thyrocervical trunks, and costocervical trunks.
Common Carotid Arteries
Branches of the common carotid arteries supply blood to the head and brain. The
right common carotid originates from the brachiocephalic trunk. The left common
carotid is the second branch of the aortic arch. At the "Adam's apple," the
common carotids divide into the external and internal carotid arteries.
The external carotid artery supplies most external head structures, except the
orbits. It gives rise to the superior thyroid artery, which
supplies the thyroid gland and larynx; the lingual artery, which
supplies the tongue; the facial artery, which supplies the skin and
muscles of face; the occipital artery, which supplies the posterior
scalp; the maxillary artery, which supplies the teeth, maxilla,
buccal cavity, external ear; and the superficial temporal artery,
which supplies the chewing muscles, nasal cavity, lateral aspect of face, most
of the scalp, and the dura mater around brain. A branch of the maxillary artery,
the middle meningeal artery, supplies the dura mater and cranial
cavity. Injury to the head that ruptures the middle meningeal artery causes
epidural hematoma, which can be fatal.
The internal carotid artery supplies the orbits and more than 80 percent of the
cerebrum. It gives rise to the ophthalmic artery, which supplies
the orbits, nose, and forehead; the anterior cerebral artery, which supplies the
medial aspect of the cerebral hemisphere, and the middle cerebral
artery, which supplies the lateral aspect of temporal and parietal
lobes. The internal carotid arteries have a carotid sinus with baroreceptors
that help regulate BP. Pressure on the neck near the carotid sinuses can cause
unconsciousness, because it simulates high blood pressure, eliciting a reflex
vasodilation and interfering with blood flow to the brain.
Vertebral Arteries
Branches of the vertebral arteries supply blood to the neck structures and the
spinal cord. They originate from the subclavian arteries at the base of the neck
and unite in the brain stem to form the basilar artery. The basilar artery
branches supply the cerebellum, pons, and inner ear. At the junction of the pons
and midbrain, the basilar artery flows into the arterial circle where it divides
into two posterior cerebral arteries that supply the occipital lobes and
inferior portions of temporal lobes.
The thyrocervical trunks supply the thyroid gland and some scapular
muscles. They are small vessels that originate from the subclavian arteries.
Costocervical trunks arise from the subclavian arteries and
supply blood to the deep neck muscles and some intercostal muscles of superior
rib cage.
Arterial Circle
The arterial circle, also called the circle of Willis, is an arrangement of
arteries surrounding the pituitary gland and optic chiasm. It creates
redundancies to ensure blood supply to the brain if one artery feeding the
circle—or a part of the circle—is damaged. The arterial circle is fed anteriorly
by the left and right carotid arteries and posteriorly by the basilar artery.
The circle itself is composed of two posterior communicating arteries, and one
anterior communicating artery. Branches off of the circle include two posterior
cerebral arteries, two middle cerebral arteries, and two anterior cerebral
arteries.
Arteries of the Thorax
The wall of the thorax is supplied by a collection of arteries, some of which
originate directly from the thoracic aorta and others from branches of the
subclavian artery. Two bronchial arteries on the left and one on the right
deliver systemic blood to the lung structures, including the visceral pleura,
esophagus, and bronchi of lungs. Blood is also delivered to the esophagus by the
four or five esophageal arteries. Numerous small mediastinal arteries deliver
blood to the posterior mediastinal structures.
Nine pairs of posterior intercostal arteries wrap around the rib cage before
anastomosing anteriorly with the anterior intercostal arteries. The first pair
arises from the costocervical trunk and the rest from the thoracic aorta. They
supply blood to the skin and subcutaneous tissue, breasts, spinal cord and
meninges, and pectoralis, intercostal, and some abdominal muscles. The
distribution of the subcostal arteries is similar to that of the posterior
intercostal arteries. They supply blood to the posterior intercostal tissues,
vertebrae, spinal cord, and deep muscles of the back. One or more pairs of the
small superior phrenic arteries supply blood to the posterosuperior surface of
diaphragm.
Internal thoracic arteries, also called the mammary arteries,
originate from the subclavian artery and supply the breast tissue. Their
branches, the anterior intercostal arteries, supply the intercostal
spaces anteriorly.
Arteries that supply the abdominal organs and abdominal wall structures originate from
the abdominal aorta. About half of resting cardiac output flows through these vessels.
The abdominal arteries are all in pairs, except for the superior and inferior mesenteric
arteries, the celiac trunk, and the median sacral artery.
Major Arteries of the Abdomen
| Artery |
Area Supplied |
Description |
| Inferior phrenic arteries |
Inferior surface of diaphragm |
Arise from the aorta just below the diaphragm |
| Celiac trunk |
Branches supply various abdominal organs |
Large unpaired artery divides into common hepatic, splenic, and left gastric
arteries; A branch of the common hepatic artery, the gastroduodenal artery,
becomes the hepatic artery proper, whose branches supply the liver;
Gastroduodenal and splenic arteries give rise to the right and left
gastroepiploic arteries, respectively, that supply the greater
curvature of the stomach |
| Superior mesenteric artery |
Branches supply the duodenum, pancreas, and parts of the small and large
intestines |
Large unpaired artery; Branches: intestinal arteries (supply most
of small intestine), ileocolic artery (appendix, cecum, and ascending colon),
right and middle colic arteries (part of transverse colon) |
| Middle suprarenal arteries |
Adrenal glands |
Adrenal glands are supplied by the middle suprarenal arteries, as
well as the superior suprarenal branches of the inferior phrenic arteries and
the inferior suprarenal branches of the renal arteries |
| Renal arteries |
Kidneys |
The left renal artery is shorter than the right |
| Gonadal arteries |
Ovaries in females and testes in males |
One artery for each gonad |
| Inferior mesenteric artery |
Branches supply parts of the large intestine |
Branches off the anterior surface of the abdominal aorta below the renal artery
branch points |
| Lumbar arteries |
Posterior abdominal wall structures |
There are four pair of these arteries |
| Median sacral artery |
Coccyx, sacrum |
Supply lower region of the abdominal cavity and base of the spine |
| Common iliac arteries |
Lower abdominal wall |
Paired arteries; Supply pelvic organs and lower limbs |
The three major arteries that supply the upper limbs are a continuing sequence of
vessels, not branches of other vessels. The axillary artery is a continuation of the
subclavian artery, and the brachial artery is a continuation of the axillary artery. The
right and left subclavian arteries send branches off to the neck. Both arteries then run
laterally between the clavicle and first rib into the axilla, where they become the
axillary arteries.
Major Arteries of the Upper Limbs
(All of the arterial supply of the upper limbs is from branches of the subclavian
arteries.)
| Artery |
Area Supplied |
Description |
| Axillary artery |
Branches supply the axilla, chest wall, and shoulder girdle |
Continuation of the subclavian artery; Branches: thoracoacromial
artery (supplies the deltoid muscle and pectoral region),
lateral thoracic artery (lateral chest wall and breast),
subscapular artery (scapula, dorsal thorax wall, part of
latissimus dorsi muscle), anterior and posterior circumflex humeral
arteries (shoulder joint and deltoid muscle) |
| Brachial artery |
Anterior flexor muscles of arm |
Continuation of the axillary artery; Most commonly used artery for routine BP
measurement; A major branch, the deep artery of the arm, supplies
the posterior triceps brachii muscle; The brachial artery divides into the
radial and ulnar arteries |
| Radial artery |
Lateral muscles of forearm; wrist; thumb and index finger |
The radial pulse can be easily palpated at the root of thumb |
| Ulnar artery |
Medial forearm; middle, ring, and little fingers; medial index finger |
Its short branch, the common interosseous artery, supplies the
deep flexors and extensors of forearm |
| Superficial and deep palmar arches |
Branches supply fingers |
The superficial and deep palmar arches are formed by anastomoses
of radial and ulnar arteries in the palm; Give rise to the metacarpal
arteries and digital arteries that supply the fingers
|
The right and left common iliac arteries are the terminal branches of the abdominal
aorta. Each of these arteries branches into internal and external iliac arteries. The
internal iliac arteries and their branches supply the pelvis, while the external iliac
arteries and their branches perfuse the lower limbs.
Major Arteries of the Pelvis and Lower Limbs
| Branch |
Area Supplied |
Description |
| Common iliac arteries |
Pelvis, lower limbs, external genitalia |
Each common iliac artery gives rise to two branches: the internal iliac and
external iliac arteries |
| Internal iliac arteries |
Pelvic wall and organs, including the bladder and rectum, uterus and vagina in
females, and prostate gland and ductus deferens in males |
Primary arteries supplying the pelvis; Branches: superior and inferior
gluteal arteries (supply the gluteal muscles), obturator
artery (adductor muscles of inner thigh), internal pudendal
artery (external genitalia and perineum) |
| External iliac arteries |
Lower limbs |
The external iliac arteries become the femoral arteries; Branches
supply the anterior abdominal wall muscles, round ligament of uterus in females,
and cremaster muscles in males |
| Femoral arteries |
Lower abdominal wall, thigh muscles, groin, external genitals |
A branch of the femoral arteries, the deep femoral artery (deep artery of the thigh) supplies most thigh muscles
(including the quadriceps, femoris, adductors, and hamstrings); The pulse of the
femoral artery can be felt just below the inguinal ligament; In a cardiac
catheterization procedure, a catheter is inserted into the femoral artery to
reach the left side of the heart |
| Popliteal arteries |
Adductor magnus and hamstring muscles, skin on the back of the legs; branches
supply calf muscles, knee joint, femur, patella, fibula |
The popliteal arteries divide into the anterior and posterior
tibial arteries; The pulse of the popliteal arteries can be felt behind the knee
|
| Anterior tibial arteries |
Knee joints, anterior compartment of the legs, skin on the front of the legs,
ankle joints; branches supply feet and toes |
At the ankle, the anterior tibial arteries become the
dorsalis pedis arteries (dorsal arteries of the
foot) that supply the joints, muscles, and skin on the dorsal part of
the foot; Each dorsal artery gives rise to arcuate arteries, which
divide into the dorsal metatarsal arteries that supply the feet;
Branches end by dividing into dorsal digital arteries that supply
the toes; Pulse in the dorsalis pedis artery is used to assess the peripheral
vascular system |
| Posterior tibial arteries |
Bones, joints, muscles of the legs and feet |
The posterior tibial arteries are a continuation of the popliteal
arteries; Divide into the medial and lateral plantar arteries |
| Medial plantar arteries |
Toes; abductor hallucis and flexor digitorum brevis muscles |
The medial plantar arteries run along the medial side of the foot
|
| Plantar metatarsal arteries |
Feet |
The lateral plantar arteries merge with a dorsal artery branch to
create the plantar arch, which gives rise to the plantar
metatarsal arteries; End by dividing into the plantar digital
arteries, which perfuse the toes |
| Fibular arteries |
Fibularis, tibialis, posterior, soleus, and flexor hallucis muscles; fibula,
tarsus, and lateral part of heel |
The fibular arteries (peroneal arteries) are major
branches of the posterior tibial arteries |
Arterial walls change with age. Arteries that undergo significant changes include the
large elastic vessels such as the aorta, the coronary arteries, and the carotid
arteries. Arteriosclerosis refers to conditions that make arteries less
elastic (commonly referred to as hardening of the arteries). This commonly occurs as we
age because of calcium deposition in the walls of vessels (calcific arteriosclerosis),
or degenerative loss of elastic fibers with age (senile arteriosclerosis).
Atherosclerosis is a specific type of arteriosclerosis that involves the
formation of plaques on arterial walls that make them stiffer. Plaques are made of fatty material containing
cholesterol. This fatty substance may eventually be replaced with connective tissue and
deposits of calcium. Plaques can rupture, initiating a blood clot at the site. Both
arteriosclerosis and atherosclerosis increase resistance to blood flow. Atherosclerosis
in blood vessels that supply the brain can reduce blood flow to the brain, causing brain
cells to malfunction or die if the reduction in flow is severe enough. Arteriosclerosis
and atherosclerosis can also lead to poor wound healing. These conditions are usually
firmly diagnosed with vascular imaging methods.
Figure 24
Figure 25
A main contributor to the development of atherosclerosis is high levels of low density
lipoprotein (LDL or “bad” cholesterol) in the bloodstream. LDL typically rises with
increased dietary saturated fat and decreased exercise. Because atherosclerosis is the
main culprit of coronary artery disease, which is a leading cause of heart disease and
death in the elderly, it is recommended that cholesterol levels be monitored with lipid
profiles conducted every five years beginning at the age of 20.
Another condition that is more common in older adults is congestive heart failure, which
is caused by impaired pumping efficiency in the heart. Congestive heart failure can
cause shortness of breath and leg swelling and is typically diagnosed by blood and
imaging tests. Treatment focuses on preventing further progression and easing symptoms
with methods such as oxygen delivery, medications, and palliative care.
Arterioles
The smallest of the vessels that carry blood away from the heart, the
arterioles, direct blood into the capillary networks. Both the
tunica interna and tunica externa of arterioles are thin and the tunica media
consists of only one or two layers of muscle cells that encircle the vessel.
However, these arterioles are particularly important in regulating the amount of
flow delivered to the tissues that they feed. These arterioles have a rich
supply of sympathetic nerve fibers. When they receive a high number of signals
from the sympathetic nervous system (i.e. a high degree of sympathetic tone),
the arterioles constrict, increasing resistance to blood flow. If the signals
from the sympathetic nervous system decrease (i.e. decreased sympathetic tone),
the arterioles dilate, reducing the resistance to flow. Arterioles can also
adjust their diameters in response to hormonal signals (such as angiotensin II)
and local signaling molecules (such as prostaglandins). Fine adjustments to the
diameter of the arteriole lumen have a direct effect on the flow of blood into
the capillary network supplied by that particular arteriole. Arterioles are
numerous and do not have individual names as the elastic and muscular arteries
do.
Figure 26
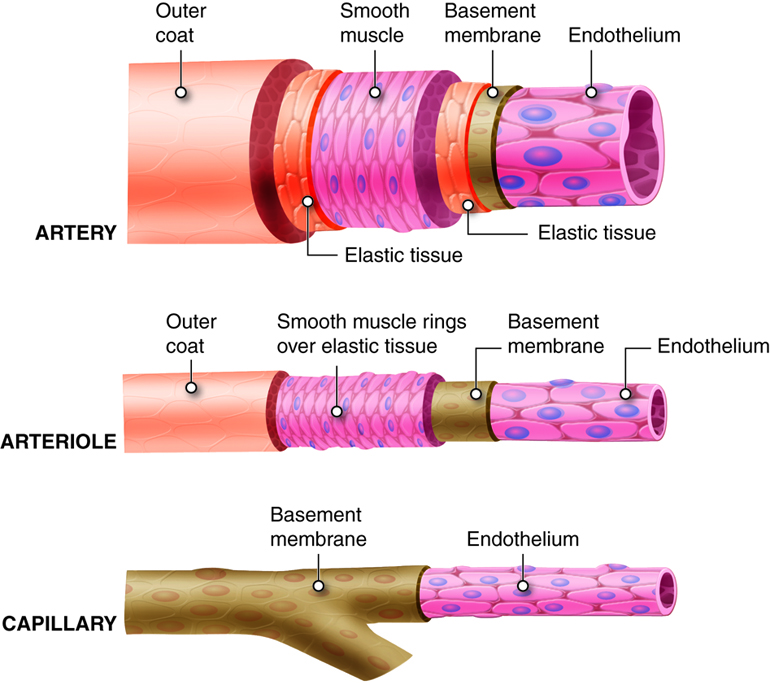
Figure 31
Metarterioles
Metarterioles are short vessels that connect arterioles to the
capillary networks. Metarterioles do not have a true tunica media. Instead, at
the metarteriole-capillary junctions a single smooth muscle cell forms a ring
around the metarteriole. Each encircling muscle cell acts as a
precapillary sphincter, regulating the flow of blood into the
capillaries that branch from the metarteriole. In response to stimuli, the
muscle cell encircling the metarteriole contracts, reducing the size of the
lumen. This prevents the flow of blood into the capillaries fed by that
metarteriole. If most or all of the precapillary sphincters associated with a
capillary network contract simultaneously, blood is moved directly from the
arterial to the venous system through the metarteriole. In this situation, the
metarteriole is acting as a thoroughfare channel, and the entire capillary
network is bypassed. Because each metarteriole regulates blood flow into a
specific number of capillaries, blood flow through any tissue is finely
controlled. Blood delivery to a particular tissue can be quickly increased,
decreased, or even temporarily halted in order to respond to the current
metabolic activity of the tissues they supply.
Figure 27
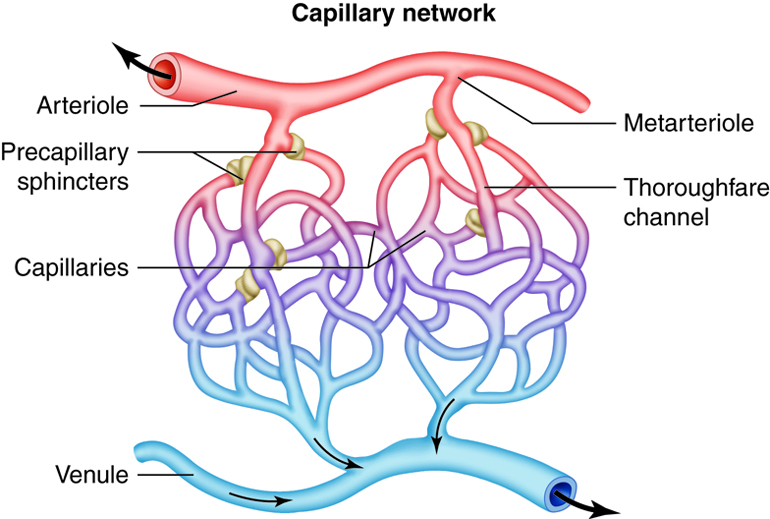
Capillaries
The smallest of all the vessels, the capillaries form extensive
networks throughout the entire body. Capillary networks form the connections
between the arterial and venous systems. The complexity of each capillary
network varies in response to the metabolic needs of the tissues served. Tissues
with high oxygen requirements, such as skeletal muscle, have 8 or 10 capillaries
branching from each metarteriole. Tissues with lower needs, such as the
intestinal tract have 2 or 3 capillaries from each metarteriole. There are even
some tissues, such as nail beds, with metabolic needs so low that the ratio of
metarteriole to capillary is one to one.
In addition to capillary network design, capillary wall construction further
facilitates the rapid exchange of gases and solutes. Capillary walls consist of
only a tunica interna. Decreasing the distance that substances must travel
increases the diffusion rate. The artery and arteriole wall thickness prevents
diffusion of gases and solutes from the blood until it arrives at the target
tissue. In some tissues the demand for oxygen and nutrients is so high that even
the thin barrier of the tunica interna prevents adequate diffusion rates. To
accommodate the specific needs of each tissue and organ, there are three
different types of capillaries, based on wall structure. Following the exchange
of gases and solute, capillary networks drain into postcapillary venules.
Types of Capillaries
Capillaries are classified by the structure and arrangement of their endothelial
cells and the underlying basement membrane. The three types, continuous
capillary, fenestrated capillary, and sinusoid
capillary, each have a characteristic structure that dictates the
level of permeability. Continuous capillaries form smooth tubes with only narrow
intercellular clefts between the adjacent endothelial cells. The basement
membrane is whole and without pores. Small molecules such as glucose and oxygen
can readily pass across and between the cells, but larger molecules such as
plasma proteins and platelets, cannot pass across the membrane. Continuous
capillaries have the lowest permeability rate of the three types and are common
in muscle, nervous, and connective tissues. The endothelial cells of fenestrated
capillaries have numerous fenestrae (“windows”). These are areas of
the cells where the plasma membranes from opposite sides of the cell adhere and
exclude the cytoplasm. Often the membrane of the fenestrations is constructed of
a material even more porous than a typical phospholipid bilayer. The basement
membrane of fenestrated capillaries has openings or pores that also increase the
permeability of the vessels. Fenestrated capillaries allow for faster solute
exchange but still exclude larger molecules from passing through. They are
common in areas that require rapid absorption or filtration such as the small
intestines, the kidneys and the choroid plexuses of the brain. Sinusoid
capillaries are limited to areas where blood cells move in and out of the
bloodstream. The endothelial cells are widely separated and contain large pores
without membrane coverings. The basement membrane of sinusoid capillaries is
sparse or missing. This structure allows large plasma proteins and even blood
cells to enter and leave the capillaries. Sinusoid capillaries are found where
blood cells are formed, such as red bone marrow, and where large proteins enter
the bloodstream, such as the liver.
Figure 28
Venules
At the venous end of a capillary network, the capillaries that branch from a
single metarteriole reunite and empty into a venule. As blood moves
into the venules, and thus the venous system, the return trip to the heart
begins. The walls of venules and veins are much thinner than those of arteries
and arterioles. Without blood within, arteries retain their shape but veins
collapse. The postcapillary venules collect blood from the capillary networks.
These smallest and most porous of the venules are involved in solute and gas
exchange in the tissues along with the capillary networks. Postcapillary venules
are also the site of white blood cell exit from the bloodstream in response to
inflammation or infection. In a reversal of the branching of arteries into
smaller and smaller vessels, venules coalesce to form larger and larger vessels,
gaining vessel wall components and thickness as they enlarge.
Figure 29
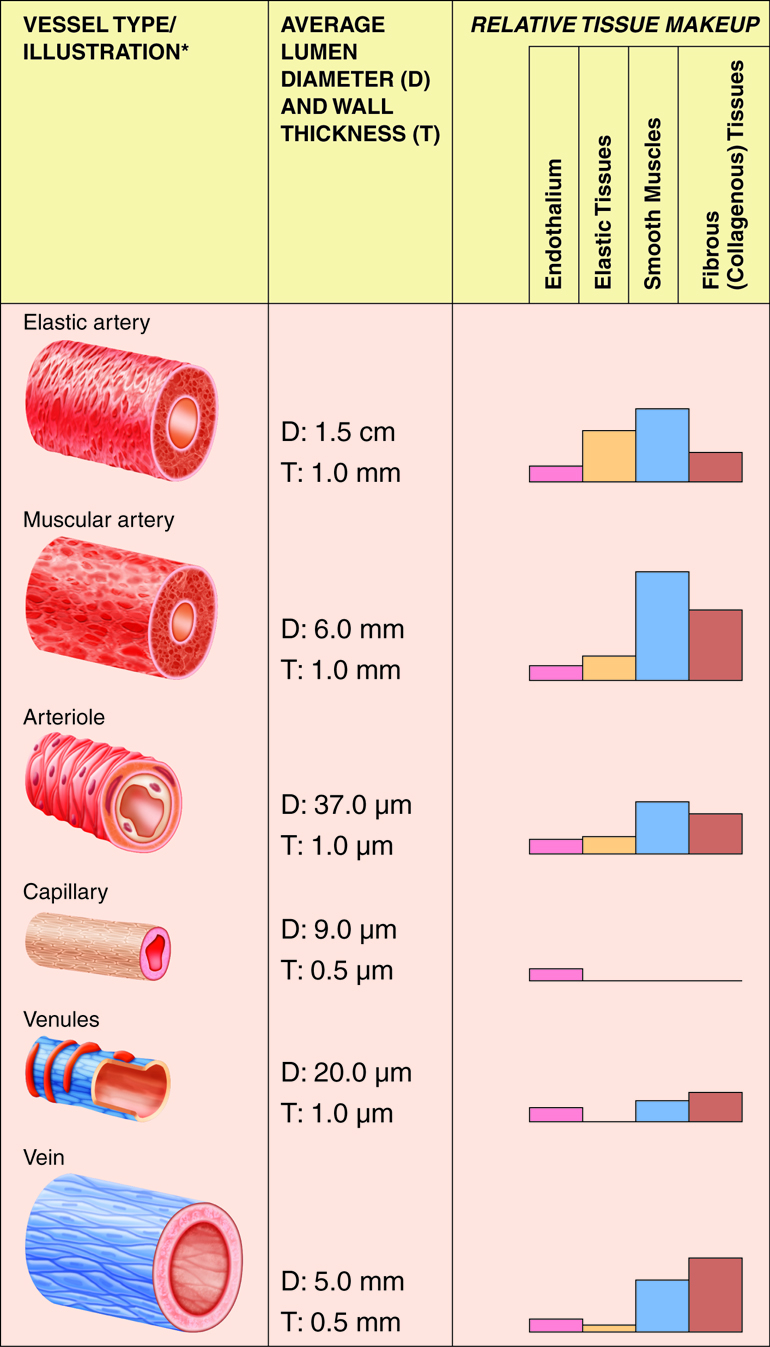
Although all three tunics are present in veins, the tunica interna and tunica media are
quite thin, and both the internal and external elastic laminae are absent, or very thin.
These features render the veins capable of great expansion to hold the variable volume
of blood passing through them. At any given time, there is three times as much blood
volume in the venous system than there is in the arterial system. However, veins are not
designed to handle the high blood pressure commonly found in arteries. Due to the
relatively large lumen and thin walls, veins will appear flattened in a micrograph since
the vessel collapses when it is not filled with blood.
Blood pressure in the venous system is much lower than blood pressure in the arterial
system (10 mm Hg compared to 90–100 mm Hg). Therefore, the flow of blood back to the
heart from the capillary networks, called venous return, cannot depend on pressure
alone. One feature of veins that augments venous return is the presence of venous
valves within the vessels, most commonly in the limbs. The valves of veins
are formed by extensions of the tunica interna that form flaps into the vessel lumen.
These valves close when blood in a vein tries to move backward, away from the heart. The
closed valve forms a barrier to the backward flow of blood.
Figure 30
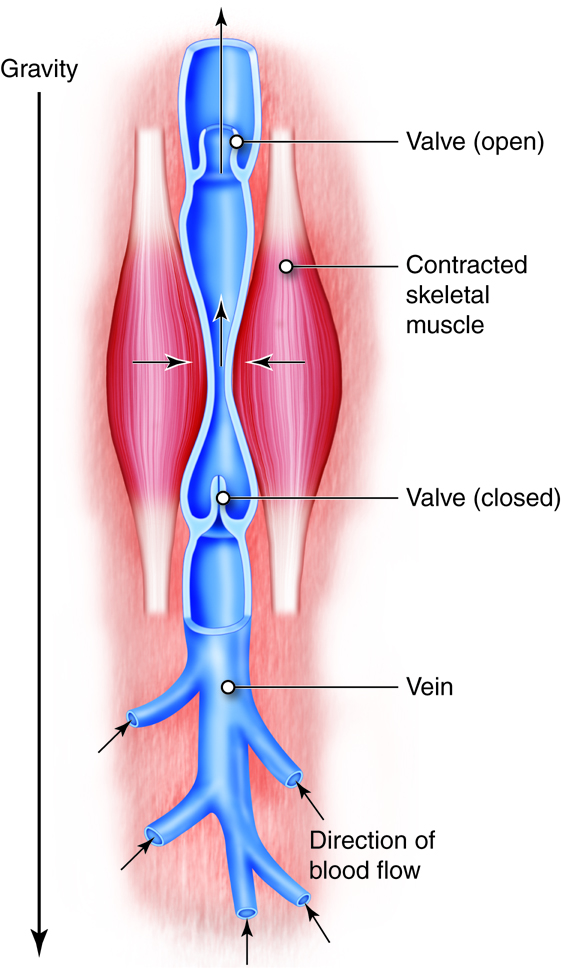
Along with the differences in wall structure, the venous system has some unique features.
One of these is the presence of venous sinuses, large-diameter veins with
extremely thin walls that contain no smooth muscle. Support is instead provided by the
dense connective tissue surrounding the vessel. Venous sinuses are collection points for
oxygen-poor blood that is headed back to the heart. Examples are the coronary sinus of
the cardiac venous system and the dural sinuses in the brain. In addition, veins are
more numerous than arteries. Often two veins will accompany a single artery. Between the
veins are connecting channels, called anastomoses. Anastomoses are most
commonly found in the limbs, and the veins that form them are not always associated with
arteries. In the upper and lower limbs, superficial veins are present where arteries are
not. Anastomoses connect the superficial veins to the deeper veins which are accompanied
by arteries. Also unique to the venous system are portal veins. Portal
veins drain one capillary network, travel to another organ or tissue, and empty into
another capillary network. The hepatic portal vein carries nutrient-rich blood from the
gastrointestinal tract and spleen to the liver.
Venous blood from the systemic circuit is returned to the right atrium by way of the
venae cavae and the coronary sinus. The superior vena cava receives veins that drain the
head, neck, chest, and upper limbs. The inferior vena cava receives veins that drain the
abdomen, pelvis, and lower limbs. The coronary sinus drains the myocardium.
The majority of blood that returns to the heart flows through the superior and inferior
venae cavae. The names of these veins are derived from the location of their
tributaries. The superior vena cava collects blood from veins located
superior to (above) the diaphragm, with the exception of the wall of the heart and the
alveoli of the lungs. The superior vena cava, which delivers blood to the right atrium,
is formed by the merger of the right and left brachiocephalic veins. Each
of these veins, in turn, is formed by the union of the internal jugular vein and subclavian vein on the right or left side of the body. The
inferior vena cava, which is the largest-diameter blood vessel in the
body, collects blood from veins located inferior to (below) the diaphragm and delivers
it to the right atrium. This vein is located directly to the right of the abdominal
aorta. Its distal end is created by the merger of the paired common iliac
veins. Blood that drains from the myocardium of the heart empties into
cardiac veins and returns to the right atrium via the coronary sinus.
| Vein |
Area Drained |
Description |
| Superior vena cava |
Head, neck, chest, upper limbs |
7.5 cm (3 in.) long, with a diameter of 2 cm (1 in.); Returns blood to the
superior portion of the right atrium |
| Inferior vena cava |
Abdomen, pelvis, lower limbs |
~3.5 cm (1.4 in.) in diameter, the widest vein in the body; Returns blood to
the inferior portion of the right atrium |
| Coronary sinus |
Myocardium |
Receives the great, middle, and small cardiac veins; Returns blood
to the right atrium; A few anterior cardiac veins drain directly
into the right atrium |
The three major vein pairs that drain blood from the head and neck are the internal and external jugular veins and the vertebral veins.
The courses
and interconnections of these veins are significantly different than
the arterial system that supplies the head. Most of the veins that
drain the brain empty into the dural sinuses. These large,
interconnected chambers lie
between the dura mater layers.
Dural Sinuses
The superior and inferior sagittal sinuses drain blood from the brain. The
superior sagittal sinus drains the cerebral hemispheres,
meninges, and cranial bones. The much smaller inferior sagittal sinus
receives blood from the great cerebral vein, which drains
deeper parts of the brain. The straight sinus is formed by the
merger of the inferior sagittal sinus and great cerebral vein. It drains the
cerebellum. The transverse sinuses drain the cerebrum, cerebellum,
and cranial bones. Near the temporal bone, the transverse sinuses become the
sigmoid sinuses, which terminate in the internal jugular veins.
The cavernous sinuses collect blood from the ophthalmic veins
that drain the orbits, the cerebral veins that drain the
cerebral hemispheres, and the facial veins that drain the nose and
upper lip. Cranial nerves III, IV, and V (ophthalmic and maxillary branches) and
the internal carotid arteries pass through the cavernous sinuses.
Jugular Veins
The internal jugular veins originate from the dural venous sinuses and drain most
blood from the brain. They collect blood from deep face and neck vein branches
of the facial and superficial temporal veins. The right and left
internal jugular veins merge with the subclavian veins to form the right and
left brachiocephalic veins.
The external jugular veins are superficial veins (right and left) that empty into
the subclavian veins. When venous pressure increases (e.g., during coughing or
conditions such as congestive heart failure), these veins bulge conspicuously at
the side of the neck. They drain the scalp, as well as superficial and deep
regions of the face. Deep structures of the neck are also drained by the
vertebral veins. Right and left vertebral veins run through transverse foramina
of the first six cervical vertebrae before exiting at the sixth cervical
vertebrae and entering the brachiocephalic veins at the base of the neck.
The brachiocephalic vein of the thorax carries blood that is on its way back from the
head, neck and upper limbs, as well as collects blood from the mammary glands and from
the first two or three intercostals spaces. The right and left brachiocephalic veins
unite to form the superior vena cava. The left brachiocephalic vein is longer than the
right because of the superior vena cava's location to the right of the body's
midline.
Most blood from the thoracic wall and tissues empties into an amalgamation of veins
called the azygos system. This system offers a collateral pathway for
draining areas served by the inferior vena cava, including the abdominal wall. A variety
of anastomoses connect the azygos system with the inferior vena cava. The veins of the
azygos system border the vertebral column laterally. Large veins draining the lower
limbs and abdomen empty into the azygos system. The azygos system can drain venous blood
from the lower body if the inferior vena cava or hepatic portal veins are damaged.
The azygos vein arises from the right ascending lumbar vein
that drains most of the right abdominal cavity wall and the right posterior
intercostal veins that drain chest muscles. It empties into the superior vena
cava. The hemiazygos vein arises from the left ascending lumbar
vein and lower posterior intercostal veins, and it ends by merging with the
azygos vein. It collects blood from the ninth through eleventh left posterior
intercostal, esophageal, mediastinal, and occasionally the accessory hemiazygos veins
and drains the left side of thoracic wall, thoracic viscera, and abdominal wall. The
accessory hemiazygos vein collects blood from the fourth through eighth
left posterior intercostal, left bronchial, and mediastinal veins. It ends by merging
with the azygos vein.
Blood from the abdominal walls and abdominal (and pelvic) organs returns to the heart via
the inferior vena cava. Blood from the digestive organs empties into veins that drain
into the hepatic portal vein. This common vessel carries the venous blood
into the liver. The hepatic veins then carry the blood from the liver to the vena cava. This hepatic portal system includes two sets of capillary beds
located between the arterial supply and the ultimate venous drainage. Capillary beds in
the stomach and intestines empty into tributaries of the hepatic portal vein, which
carries the blood to the capillary bed in the liver. The hepatic portal system also
includes a variety of tributary vessels from the stomach and pancreas, including the
superior and inferior mesenteric veins and the splenic vein. The superior
mesenteric vein collects blood from all of the small intestine, the ascending
and transverse colons of the large intestine, and the stomach. The splenic vein drains the spleen and portions of the pancreas and stomach. This vein unites with
the superior mesenteric vein to create the hepatic portal vein. Blood from the distal
parts of the large intestine and rectum drains into the inferior mesenteric
vein, which unites with the splenic vein immediately prior to its merger with
the superior mesenteric vein to form the hepatic portal vein.
Major Veins of the Abdomen
| Vein |
Area Drained |
Description |
| Inferior vena cava |
Abdomen, lower limbs, pelvis |
Formed by the merger of the two common iliac veins |
| External iliac veins |
Abdominal wall, lower limbs, cremaster muscle in males |
Companions of the internal iliac arteries; Continuations of the femoral veins;
End by merging with the internal iliac veins to form the common iliac veins
|
| Lumbar veins |
Posterior abdominal wall (both sides), vertebral canal, spinal cord,
meninges |
Usually four lumbar veins on each side; Some drain directly into
the inferior vena cava and others into the ascending lumbar veins of the
thoracic azygos system |
| Gonadal veins |
Testes in males; ovaries in females |
Gonadal veins are called testicular veins in males and
ovarian veins in females; Left testicular and left ovarian
veins drain into the left renal veins; Right testicular and right ovarian veins
empty into the inferior vena cava |
| Renal veins |
Kidneys |
The right renal vein is shorter than the left; The left renal vein
collects blood from the left ovarian/testicular, left inferior phrenic, and left
suprarenal veins; The right renal vein drains into the inferior vena cava |
| Suprarenal veins |
Adrenal glands |
The left suprarenal vein drains into the left renal vein; The
right suprarenal vein drains into the inferior vena cava |
| Inferior phrenic veins |
Diaphragm |
Typically, the left inferior phrenic vein has tributaries to the left
suprarenal vein (which drains into the left renal vein) and to the inferior vena
cava; The right inferior phrenic vein drains into the inferior vena cava |
| Hepatic veins |
Liver |
Right and left hepatic veins empty into the inferior vena cava |
The deep veins of the upper limbs have similar courses and names as their companion
arteries. The superficial veins are not paired with arteries, but provide an anastomotic
network for draining the limbs. The large superficial veins in the upper limbs are visible just below the skin.
Major Veins of the Upper Limbs: Deep
(Some veins of the upper limbs also drain structures of the thorax.)
| Vein |
Area Drained |
Description |
| Radial veins |
Lateral side of forearms |
Paired radial veins begin at the deep palmar venous arches that drain the palmar metacarpal veins in the palms |
| Ulnar veins |
Medial side of forearms |
Paired ulnar veins begin at the superficial palmar venous arches,
which drain the common palmar digital veins and proper palmar
digital veins in the fingers |
| Brachial veins |
Forearms, elbow joints, upper limbs, humerus |
Paired brachial veins merge with the basilic veins to
form the axillary veins
|
| Axillary veins |
Upper limbs, axillae, superolateral chest wall |
Continue as the subclavian veins |
| Subclavian veins |
Upper limbs, neck, thoracic wall |
Continuations of the axillary veins; Merge with the internal jugular veins to
form the brachiocephalic veins |
Major Veins of the Upper Limbs: Superficial
(Some veins of the upper limbs also drain structures of the thorax.)
| Vein |
Area Drained |
Description |
| Cephalic veins |
Lateral side of upper limbs |
The cephalic veins begin on the lateral side of the dorsal
venous arch, a plexus of veins formed by the dorsal metacarpal
veins that drain the dorsal digital veins of the fingers
|
| Basilic veins |
Medial aspects of upper limbs |
At the crook of the elbow, the basilic veins are connected to the cephalic
veins by the median cubital vein that drains the forearm; The
median cubital vein is often used to draw blood or inject intravenous
medications; In the axilla, the basilic veins merge with the brachial veins to
form the axillary veins |
| Median antebrachial vein |
Palms, forearms |
The median antebrachial vein runs between the radial and ulnar
veins in the forearm; Ends at the elbow by entering either the basilic or
cephalic vein |
Most of the veins that drain the pelvis and lower limbs have the same names as the
arteries they accompany. All of these veins empty into the inferior vena cava, which
returns the blood to the heart.
Major Veins of the Pelvis and Lower Limbs: Deep
| Vein |
Area Drained |
Description |
| Posterior tibial vein |
Feet, muscles of the posterior compartment of the lower leg |
The posterior tibial vein is formed by the merger of the medial
and lateral plantar veins; Receives the fibular vein
(peroneal vein) |
| Dorsalis pedis vein |
Foot |
The dorsalis pedis vein becomes the anterior tibial
vein
|
| Anterior tibial vein |
Ankle joint, knee joint, tibiofibular joint, anterior portion of leg |
Paired veins; Accompany the anterior tibial arteries; Merge with the posterior
tibial vein at the knee to form the popliteal vein
|
| Popliteal vein |
Knee joint; skin, muscles, and bones of parts of the calf and thigh near the
knee joint |
Collect blood from the small saphenous veins and tributaries that correspond to
popliteal artery branches |
| Femoral vein |
Deep thigh muscles, femur |
The femoral vein is a continuation of the popliteal vein; Collects
blood from the deep veins of the thigh (deep femoral
veins) and great saphenous veins; Called the external
iliac vein when it ascends into the pelvic cavity |
| External iliac veins |
Lower limbs, cremaster muscle in males, abdominal wall |
The external iliac veins are continuations of the femoral veins;
In the pelvis, they merge with the internal iliac veins to form the common iliac
veins |
| Internal iliac veins |
Pelvis, thigh, buttocks, external genitalia |
In the pelvis, the interior iliac veins merge with the external
iliac veins to form the common iliac veins |
| Common iliac veins |
Pelvis, external genitalia, lower limbs |
Formed by the merger of the internal and external iliac vein; The left common
iliac vein is much longer than the right; The two common iliac veins join to form the inferior vena cava |
Major Veins of the Pelvis and Lower Limbs: Superficial
| Vein |
Area Drained |
Description |
| Great saphenous veins |
Medial side of leg and thigh, groin, external genitalia, abdominal wall |
Longest veins in the body; Begin at the dorsal venous arches; The great
saphenous vein is often harvested for use as a coronary bypass vessel |
| Small saphenous veins |
Deep fascia of calf muscles |
At the knee, the small saphenous veins empty into the popliteal
veins |
Cardiac output is homeostatically maintained throughout our lifetime so that it
constantly meets the needs of the body’s tissues. When the heart becomes damaged, such
as after a heart attack, it may not be able to maintain adequate flow. This causes blood
pressure to fall, initiating homeostatic feedback loops to try to bring blood pressure
(and cardiac output) back to normal.
One way that the heart can become damaged is through a heart attack. This occurs when
coronary blood vessels, which supply the cells of the heart itself with oxygen and
nutrients, become significantly narrowed or completely blocked with a combination of
plaque and thrombus (clot). During a heart attack, cells lack oxygen, causing them to
die. If enough heart muscle cells die, the heart weakens so that it can’t pump as much
blood, leading to the various negative effects associated with this condition.
Congestive heart failure is another common condition affecting cardiac output. It may
develop after someone has a heart attack, or as a consequence of many other cardiac
conditions. The problem with congestive heart failure is that the heart muscle is
weakened to the point that it doesn’t do a good job pumping out the blood that is
flowing into it (the blood flowing into the heart is called venous return). In fact, one
of the main feedback loops that regulates blood pressure does so by adjusting the amount
of blood in the body. This is because the more blood we have, the greater our venous
return, and the higher the cardiac output – assuming a healthy heart. With the weakened
heart associated with congestive heart failure, more venous return can actually lead to
less cardiac output. A lowered cardiac output decreases blood pressure, signaling the
body to increase blood volume, increasing venous return, and making cardiac output lower
still. Because this loop continually makes the problem with cardiac output and blood
pressure worse, it is a positive feedback loop – and not one we want to experience.
We have discussed mechanisms within the heart that regulate cardiac output. For example,
an increased heart muscle contraction will increase with an increase in venous return.
Thus the healthy heart can pump more blood when there is more blood to pump.
There are other controls of heart function and cardiac output from the nervous system and
endocrine system, which we will discuss in the integration of systems. It is important
to note that we do not consciously change our heart rate or cardiac output. Instead we
can indirectly adjust the nervous and endocrine feedback loops by initiating activities
such as exercise or relaxation techniques.
Homeostatic Imbalances of the Plasma Constituents
Although most people realize the importance of red blood cells in distributing
oxygen to the body’s tissues, it is important to realize that the plasma
(non-cellular) fraction of blood is also critically important. One function of
the plasma is to form blood clots when blood vessels are cut or torn in order to
prevent excessive blood loss. Blood clotting needs to be limited to the vessels
that are damaged in order for the system to function appropriately.
Disseminated intravascular coagulation (DIC) is a serious
condition in which the clotting cascade becomes activated in many non-injured
blood vessels at one time. This occurs secondary to a significant insult to the
body such as burns, cancer, infections, etc. With this generalized activation of
the clotting cascade, small blood clots are formed, some of which limit or block
off the flow of blood within vessels. This cuts off the flow of oxygen to
organs, and if prolonged, can results in tissue necrosis (cell death), and the
organ may stop functioning altogether. The random coagulation throughout the
body leads to the coagulation proteins, as well as platelets, being used up,
lowering the concentration of the essential clotting factors in the plasma. Once
the coagulation process is disrupted, the patient suffers from abnormal bleeding
from GI tract, respiratory tracts, and from the tiniest wounds in the
body.
Homeostatic Imbalances of Red Blood Cell Concentration
Red blood cell concentration is sensitive to changes in oxygen levels. Prolonged
exposure to low oxygen causes increased production of RBCs, raising the
concentration of red blood cells in the blood (known as hematocrit), in order to
maintain homeostatic oxygenation of the tissues. However, hematocrit levels that
get too high can also lead to dysfunction, leading to a condition called
polycythemia. With polycythemia, the blood will have increased
viscosity, making it thicker and harder for the heart to pump this blood through
the body. This can lead to heart failure in severe cases. Polycythemia can
either be primary, where it is associated with an abnormality in the bone marrow
that causes overproduction of red blood cells, or secondary, where red blood
cell production is normally stimulated, but continues to the point of developing
polycythemia.
An example of secondary polycythemia is chronic mountain sickness (Monge’s
disease), which is an uncommon condition that occurs in certain people living at
high altitudes (above 12,000 feet) for months or years. At higher altitudes, the
atmospheric pressure decreases, resulting in less oxygen availability due to
lower partial pressure of oxygen in the atmosphere. This stimulates RBC
production to bring people to a slightly higher hematocrit, which then levels
off. In those that develop polycythemia, their RBC production continues over
time, making their hematocrit very high. As mentioned previously, as their
hematocrit increases, so does blood viscosity. An increase in viscosity makes it
more difficult for blood to move through blood vessels and results in the heart
pumping vigorously to get blood circulating through the body. This increased
resistance of blood places an excessive strain on the heart that may lead to
inadequate pumping of blood and decreased oxygenation of tissue. This can lead
to cardiac ischemia and hypertension due to the body’s effort to return to
homeostasis. Treating chronic altitude illness requires the removal of blood
(about a pint) as a form of temporary relief. However, the only effective form
of treatment is to descend to a lower altitude.
Homeostatic Imbalances Caused by Vessel Dysfunction
The tissues throughout the body regulate flow to themselves by sending signals
that can constrict or dilate the blood vessels that “feed” them. If these
vessels don’t have an ability to make the adjustments, the tissues won’t be able
to appropriately match blood flow with their metabolic activity.
One example of this is stable angina, where people experience chest pain on
exertion. Stable angina is associated with atherosclerotic plaque that has
developed in the main coronary arteries, which deliver flow to the cardiac
muscle. Because of the plaque, these vessels can’t dilate appropriately when the
needs of the heart muscle increases. Any type of increase in physical activity
is not met with the normal coronary artery vasodilation in these individuals.
This leads to low oxygen levels in their tissues, which can cause pain, as well
as prevent the heart from functioning optimally, limiting their activity
level.
Autoregulation refers to a tissue's ability to meet its
metabolic needs by automatically altering its blood flow. Autoregulation is
particularly important in organs such as the heart and skeletal muscles, whose
need for oxygen, nutrients, and the removal of wastes during exercise can be up
to 10 times higher than when the body is at rest. In addition to increasing perfusion
in the heart and muscles during physical activity, autoregulation manages regional blood
flow to other tissues as well, including the brain. Individual mental and physical tasks
require significantly different patterns of blood distribution. When a person is on the telephone,
for example, motor speech areas of the brain receive more blood when talking, and auditory areas
get more blood flow when listening. Autoregulatory changes in blood flow occur in response to
local signaling molecules that cause constriction or dilation of local arterioles.
Vasoconstrictors and Vasodilators
The diameter of blood vessels can be changed by chemicals secreted by various cells, such as white blood cells,
smooth muscle cells, platelets, endothelial cells, and macrophages. Metabolically active tissue cells can release
vasodilating chemicals such as potassium ions, hydrogen ions, lactic acid, and adenosine. Endothelial cells can also
release the vasodilator nitric oxide. Tissue that is injured or inflamed secrete vasodilating compounds of the
prostaglandin family. Chemicals that cause vasoconstriction include serotonin (released from platelets) and endothelins
(from endothelial cells).
Changes in local oxygen concentrations elicit two very different
autoregulatory reactions in the pulmonary and systemic circulations.
In the systemic circulation, decreased oxygen levels in the tissues
cause blood vessels to dilate, increasing the delivery of oxygen.
In the pulmonary circulation, low oxygen levels in poorly
ventilated alveoli cause the blood vessels feeding those alveoli to
constrict.
As a result, blood avoids most of the alveoli in the lungs that
have poor ventilation. Instead, most blood flows to well-ventilated
alveoli.
Our cardiac output varies with our level of activity, decreasing when resting and
increasing when we are more active. For example, cardiac output can increase from the
typical 5 L/min at rest up to 15-20 L/min during maximal exercise. When we experience
such changes in cardiac output it is important to realize that not all organs and
tissues will experience similar changes in blood flow. So when your cardiac output
triples, you will not experience a tripling of flow to each organ. Instead, flow is
directed to those tissues that need it most, typically based on their metabolic state.
If you are sprinting, your cardiac output might triple, but the flow to the muscles in
your legs might go up six or eight fold, while flow to your digestive organs can
actually decrease. Thus flow is directed to those capillary beds surrounded by cells
with the greatest requirement for oxygen and nutrients.
There are some organs in the body where their level of blood flow is not as dependent on
their metabolic need, but because they are important in processing and/or interacting
with blood in other ways. Examples include the kidneys, which process the blood by
removing metabolic wastes, the lungs, which participate in gas exchange, the liver,
which processes the substances absorbed in the GI tract, glands, which release hormones
into the bloodstream, and the skin, which helps regulate body temperature. In the case
of the liver and the pituitary gland, they interact with the blood through a portal
system, where the vascular network in these tissues contains two capillary beds along
the path of blood flow. The kidney blood flow also involves two capillary beds, but
these are connected by an arteriole.
The portal system involving the liver plays an important role in processing substances
absorbed by the capillaries along the intestines. These capillary beds in the intestines
absorb nutrients and other chemicals. This same blood then travels to the liver where it
enters the liver sinusoids (a second capillary bed) before returning to the venous
circulation. Specific functions occurring in the liver include storing glucose as
glycogen, repackaging lipids into lipoproteins and detoxification of harmful, and even
useful, chemicals, such as drugs. In fact, when discussing the ability of orally
administered drugs to distribute throughout the body, the effect of the liver is known
as the “first pass” effect.
The kidney actually contains two capillary beds in the same organ, where the first
capillary bed (the glomerular capillaries) filters the blood and the second (either the
peritubular or vasa recta capillaries) reabsorbs most of the original filtrate.
The cells of the anterior pituitary gland respond to chemical signals secreted into
capillaries of the hypothalamus. Once the blood from the hypothalamus enters the
anterior pituitary, it enters a second capillary bed so that the cells of the anterior
pituitary can sense these signals and respond by secreting their own hormones if
necessary.
As you have learned, homeostasis relies on communication between body systems, which is
primarily carried out by the nervous and endocrine systems. To convey information, the
nervous system uses neural impulses, and the endocrine system uses hormones released
into the blood. Most homeostatic controls involve negative feedback mechanisms, in which
the output of a process either terminates or lessens the original change.
Neural Regulation of Cardiac Output
Both the parasympathetic and sympathetic branches of the autonomic nervous
system (ANS) alter the cardiac output as necessary. The ANS modulates
the heart rate and force of contraction (contractility) of the
myocardium.
The parasympathetic nervous system decreases the heart rate. The
vagus nerve (the 10th cranial nerve) innervates the SA and AV
nodes of the heart. It is the parasympathetic branch of the ANS that sets the
heart rate under normal resting condition. This is commonly referred to as the
“vagal tone” as it is the vagus nerve that is responsible for this basal cardiac
function. The effect of the parasympathetic system is to decrease the rate at
which action potentials are generated at the SA and AV nodes and also to slow
down the conduction speed between the SA and the AV nodes. Increasing
parasympathetic activity increases the P-R interval of the ECG. Because the
parasympathetic nervous system does not innervate the ventricular cells, it does
not significantly affect the level of contractility of the ventricular
myocardium. Conversely, the heart rate is increased through the actions of the
sympathetic nervous system. The sympathetic nerves run from the
sympathetic chain ganglia, found along the thoracic region of the spinal cord to
the SA node, the AV node, and the myocytes of the ventricles. The sympathetic
nerves increase the heart rate by increasing the spontaneous depolarization rate
of the SA and AV nodes, and increases the rate of conduction through the AV
node. Sympathetic innervation of the ventricular myocytes can be used to
increase the contractility of the heart.
The rate of action potential generation is called chronotropy. As we
discussed above, the parasympathetic branch produces negative chronotropy. The
vagus nerve releases acetylcholine, which binds to muscarinic receptors of the
SA and AV nodal cells. When engaged, receptors activate a signal transduction
pathway that ultimately leads to the opening of potassium channels. As these
potassium channels remain open, potassium moves down its electrochemical
gradient and out of the cell. This results in hyperpolarization of the nodal
cells, leading to delayed or slower rate of depolarization. Thus the effect is
to decrease the heart rate (negative
chronotropy).
In contrast, the sympathetic nervous system exhibits a positive chronotropic
effect. Sympathetic nerves release norepinephrine (NE), which is also called
noradrenaline, that binds Beta1 receptors in the nodal cells and cardiac
myocytes.
Binding of NE (or circulating epinephrine) to the Beta-1 adrenergic receptors
activate the signal transduction pathway that ultimately leads to the opening of
calcium channels, allowing influx of Ca++. Calcium channel activation on the
nodal cells leads to positive chronotropy. Binding of NE (or circulating
epinephrine; Epi) to the Beta-1 adrenergic receptors activates the signal
transduction pathway that ultimately leads to the opening of calcium channels,
allowing influx of Ca++, which increases the contractility of the cardiomyocytes
(positive inotropy). Calcium channel activation on the nodal cells also leads to
positive chronotropy.
Changing the contractility of the ventricular muscle also influences the overall
cardiac output. This change in contractility is called the inotropic effect.
Binding of NE to Beta1 receptors of cardiomyocytes increases the contractility
(positive inotropy) by allowing a greater calcium concentration in the cytosol,
leading to a greater number of crossbridges being formed.
Before the sympathetic system may exert its effect, inhibition of the vagus
nerve is required.
A number of hormones are involved in the regulation of blood flow and BP. They work by
changing cardiac output, systemic vascular resistance, or total blood volume.
The Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system is set in motion when
decreases in blood pressure or renal blood flow stimulate cells in the kidney to
secrete renin into the blood. This leads to a pathway that
ultimately produces the hormone angiotensin II, which increases BP
in two ways. First, as a vasoconstrictor, angiotensin II increases BP by
increasing peripheral vascular resistance. Second, it causes the kidney tubules
to reabsorb sodium. High plasma angiotensin is also a trigger for higher levels
of aldosterone synthesis by the adrenal cortex. This hormone also
acts on the kidneys to increase the reabsorption of sodium ions. The increased
reabsorption of Na+ leads to enhanced water reabsorption at the kidneys, and
this causes the total blood volume to rise, elevating the BP and leading to an
increase in venous return. This increase in the amount of blood flowing into the
heart also increases cardiac output.
Epinephrine and Norepinephrine
Sympathetic stimulation promotes the release of epinephrine and
norepinephrine by the adrenal medulla. As described above,
these hormones (or neurotransmitters) increase the heart rate (chronotropy) and
force of contraction (inotropy). As the force of contraction increases the
stroke volume also increases. Because cardiac output (CO) is the product of HR
and SV, CO increases. The increase is CO will also lead to an increase in
arterial pressure, as described earlier. Epinephrine (or norepinephrine) also
has the effect of constricting arterioles and veins in the abdominal organs and
skin, while dilating vessels in the heart and skeletal muscle. This results in
increased blood flow to muscles during physical activity.
Antidiuretic Hormone
Decreased blood volume or dehydration prompts an increased release of
antidiuretic hormone (ADH) from the posterior pituitary. This
hormone, which is produced by the hypothalamus and stored in the posterior
pituitary, causes the kidney to retain water. This retention can lead to an
increase in blood volume and increasing preload similarly to the
renin-angiotensin system. At very high levels (such as those released during
circulatory shock) it also causes peripheral blood vessels to constrict, helping
to raise the BP. This is why antidiuretic hormone is also known as
vasopressin.
Atrial Natriuretic Peptide (ANP)
Atrial natriuretic peptide (ANP), which is secreted by the myocytes
of the atria of the heart, decreases BP in two ways. First, it dilates blood
vessels. Second, it reduces blood volume by increasing the excretion of salt.
This is a peptide hormone that is secreted in response to higher blood volume
that stretches the atrial walls. The effect of ANP is to ultimately lower blood
pressure. To achieve that, ANP facilitates glomerular filtration pressure,
increases excretion of sodium and urea, inhibits aldosterone secretion, and
inhibits the renin-angiotensin system.
The activity below consists of several sections of content and
questions that involve various scenarios that may arise for a patient
visiting their physician for cardiovascular related problems.
Explore the following activity to understand how various
cardiovascular related changes would be identified, as well as how they
would result in other adverse effects throughout the body.
The activity consists of three sections.
- The top left panel contains an image with hotspots. Use this for
navigation. Each hotspot connects to a section of unique material and
activities.
- The top right panel changes each time you choose a new section to
work through. Look to this panel for the information you will need to
solve the problems and questions that also appear for that section.
- The bottom panel changes each time you choose a new section as well.
You will find activities and questions here that are unique to that
section.
Be sure to click on each hotspot to ensure you complete all of the
questions within this activity. When you are sure you have finished each
section, complete the follow-up activity directly below this one.
Gathering Information about the Cardiovascular System
Introduction
Introduction
There are many tools and tests that doctors use to gather
information that indicates what is going on in the physiology of their
patients.
In this activity, we explore several common tests and tools
used to gather information about the state of the anatomy and physiology
of the Cardiovascular system. Throughout the activity, you will have
opportunities to evaluate the information that they provide and tie this
back to what you have learned about how the Cardiovascular System works
Directions: Using the various diagnostic tests and
instruments available, consider likely causes and how the information
that each diagnostic test provides can be used support or rule out
likely causes.
Blood Tests and Blood Chemistry
Blood tests are ways to determine the chemical composition of
the blood. Many cardiovascular diseases have been correlated with
altered blood chemistry. As examples, high levels of cholesterol and/or
triglycerides increase a person’s risk for heart attack and stroke,
whereas elevated levels of fibrinogen or C-reactive protein might
indicate that atherosclerotic plaque has formed in the coronary or
cerebral vessels. Other chemicals, including homocysteine and
lipoprotein A, have been found to be elevated in heart disease. It is
important to note that in some cases the mechanisms that connect
chemistry and organ performance are poorly understood.
| |
measured |
normal |
abnormal |
| cholesterol (mg/dL) |
208 |
<200 |
>240 |
| LDL (mg/dL) |
98 |
<100 |
>160 |
| HDL (mg/dL) |
110 |
>80 |
<40 |
| triglycerides (mg/dL) |
130 |
<150 |
>200 |
| C reactive (mg/L) |
0.9 |
<1 |
>3 |
| fibrinogen (mg/dL) |
258 |
<300 |
>460 |
| homocystein (uM) |
8 |
<10 |
>14 |
| lipoprotein A (mg/dL) |
11 |
<14 |
>19 |
Cardiac MRI
Magnetic resonance imaging (MRI) is used to image soft
tissues within the body. Briefly, a large magnetic coils align protons
in the direction of the magnetic field. As the protons relax back to
their unaligned state, a detector is able to record the density of the
material that the protons are found in. Contrast agents (including
gadolinium) can be added intravenously (injection into the veins) to
enhance contrast within blood vessels.
The heart never stops beating, and it can be difficult to
capture image movements using MRI. To image the heart by MRI special
procedures are used for rapid acquisition of sections. As such, cardiac
MRIs are able to image anatomical features (not physiological functions)
including congenital defects, vessel abnormalities and tumors.
A radiologist will interpret the MRI images to determine if
anything is abnormal. A doctor will discuss the results with the
patient.
Blood Pressure
Blood pressure is a measure of the pressure exerted by the
blood on the vessels. High blood pressure, also called hypertension, is
correlated with heart disease and vascular problems. The high pressure
on the vessels leads to damage and contributes to the development of
atherosclerotic plaques. Hypotension, or low blood pressure, can lead to
dizziness or fainting.
Blood pressure can be measured quickly and easily using a
sphygmomanometer (inflatable blood pressure cuff). Manual
sphygmomanometers require a stethoscope to hear the sounds of the
turbulent blood flowing through the brachial artery of the arm. The
sphygmomanometer is placed on the upper arm at the same level as the
heart, and the stethoscope is placed on the brachial artery of the
elbow. The cuff is inflated so that there is no flow that can be heard
in the brachial artery. The examiner slowly releases the pressure, and
the "whooshing" sound during the first blood flow occurs at a the
systolic blood pressure. Pressure is further released until no sounds
are heard at the diastolic blood pressure.
Blood Pressure Measurement (BP)
| |
systolic |
diastolic |
| normal (mmHg) |
90-119 |
60-79 |
| measured (mmHG) |
95 |
70 |
Heart Auscultation
Auscultation is obtaining information through listening. A
stethoscope is a medical acoustic device commonly used for auscultation.
When pressed against the chest, a stethoscope can allow someone to hear
the closing of the heart valves. A stethoscope may also allow one to
hear turbulence of blood flow rushing into chambers or squeezing through
narrowed valves in a heart.
Normal S1 S2 Heartbeat
With a stethoscope, a physician can listen to the rate and
regularity of the heartbeat. Valvular disorders, including regurgitation
(where blood leaks backward through a valve), can be heard with a
stethoscope.
The following video exemplifies the sounds associated with a
specific abnormality, Mitral Regurgitation which is a type of heart
murmer.
Mitral Regurgitation (murmer)
ECG
An electrocardiogram (ECG; EKG) is a device that uses
electrodes to measure the distribution of electrical charges around the
heart. The charge distribution changes as the action potentials
(impulses) travel through heart, producing the ECG tracing. This tracing
(recording) provides information about the rate and regularity of
heartbeats, and can also be used to measure irregularities in cardiac
conduction.
The output of the ECG for a cardiac cycle is a P wave, a QRS
complex and a T wave. The P wave is initiated by the “firing” of the SA
node and the subsequent depolarization of the atrial cells. The QRS
complex is the depolarization of the ventricles; during which time the
repolarization of the atria is obscured. The T wave is the
repolarization of the ventricles.
ECGs record tens to hundreds of cardiac cycles. Cardiac
defects often show up as minority events. Also, the spacing between
cardiac cycles and between waves of a single cycle can also reflect
cardiac disorders.
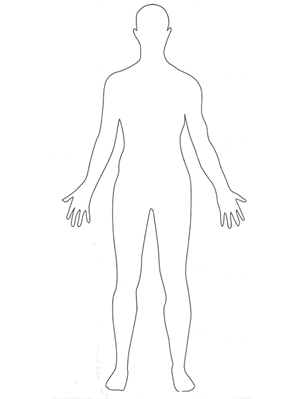
Major Function of the Respiratory System
The major function of the respiratory system is to obtain oxygen and remove
carbon dioxide for metabolic processes in the body. The aerobic metabolic
pathway of conversion of nutrients to energy requires oxygen, for example:
So, for each molecule of glucose that is converted to energy, a molecule of
oxygen is needed. Because the cells require a continuous supply of ATP, the
body, in turn, needs a constant intake of oxygen and method for removal of
carbon dioxide. Our respiratory system functions to accomplish these necessary
gas exchanges.
- Respiration
-
(Definition)
Respiration is the transport of oxygen from the air to the cell in
the tissue, and the removal of carbon dioxide in the opposite
direction.
Upper Respiratory Tract
Nose
The external portion of the nose begins at the base of the frontal bone
and extends over the maxilla, with the nasal bone providing the bridge
of the nose. Extending from the nasal bone is a collection of hyaline
cartilages that make up the bulk of the nose. The medial region of the
nose consists of a central septal cartilage with two lateral processes.
The tip of the nose contains the major alar cartilage. Two minor alar
cartilages are found at the sides and base of the lateral septal
cartilages. Dense fibrous connective tissue is found under the skin of
the sides lateral aspects the nose, away from the cartilage. Variations
in the size of a person’s nose, or its form, are due to differences in
the various cartilages.
The visible nose is actually the entryway into the nasal cavity, where
the major functions of the nose occur. The openings to the nose, the
nares, are lined with coarse hairs to aid in filtration of particulate
matter. The area immediately inside the nares, the vestibule, contains a
large number of sebaceous glands, sweat glands, and hair follicles.
The nasal cavity is divided into right and left sides by the nasal
septum. This dividing wall’s anterior portion is made of cartilage; bone
contributed by the vomer and part of the ethmoid bones of the skull make
up the posterior. The roof of the nasal cavity consists of parts of the
ethmoid and sphenoid bones. Its floor, the palate, forms the roof of the
mouth. It is separated into the hard and soft palate. The anterior hard
palate is formed from the maxillary process of the palatine bone. The
posterior soft palate does not contain bone and moves during swallowing
to close off the nasal cavity to prevent material from entering it from
the mouth.
Extending from the nasal septum are three pairs of C-shaped structures
called conchae. The superior, middle, and inferior conchae
extend the length of the nasal cavity. They are covered by a mucus
membrane that contains a large number of mucus-secreting cells and blood
vessels. A bloody nose highlights the richness of the blood supply to
the membranes on the conchae. The conchae serve as baffles to increase
the surface area of the nasal cavity. The mucus glands and blood vessels
aid in humidifying and warming the air coming into the body.
There are two types of epithelial coverings in the nasal cavity:
respiratory epithelium and olfactory epithelium. Both types of epithelia
appear very similar when viewed through a light microscope. The
olfactory mucosa found in the roof of the cavity detects odors. This
epithelial layer contains specialized nerve cells that detect odors and
transmit impulses to the first cranial nerve. The
respiratory epithelium that covers the rest of the
nasal cavity is also found through most of the respiratory tract and is
pseudostratified, ciliated, columnar epithelia.
Paranasal sinuses
Within the bones surrounding the nasal cavity are paranasal
sinuses (a sinus is a hollow area), which function to make
the skull lighter as well as moisten and warm incoming air. These
sinuses frequently become filled with excess fluid when a person has a
head cold. Since the paranasal sinuses serve as resonators for speech and
sound it is not surprising that the sound of the voice becomes altered
when they are filled with fluid or swollen. The frontal, sphenoid,
ethmoid and maxillary bones all contain sinus cavities.
Pharynx
As the air passes posteriorly through the nasal cavity, it enters the
pharynx. This structure encompasses three distinct areas and connects
the nasal passage to the larynx in the throat. It extends about 13
centimeters or 5 inches from the base of the skull to the level of the
sixth cervical vertebrae. The wall of all three portions contains two
layers of skeletal muscle. The inner layer is arranged in a circular
pattern, and the outer layer is arranged longitudinally.
-
Nasopharynx
The superior section of the pharynx is called the nasopharynx. It
is posterior to the nasal cavity and inferior to the sphenoid
bone. This chamber shares the same epithelia as the nasal cavity
and acts only as a conduit for air. The other two portions of
the pharynx contain shared passages with the digestive tract and
act as conduits for both air and food. The nasopharynx closes
off during swallowing by raising the soft palate. Also found in
this area are structures associated with immune system. The
paired pharyngeal tonsils, also known as the adenoids, lie in
the posterior wall of the nasopharynx.
-
Oropharynx
The second portion of the pharynx is the oropharynx. It runs from
the soft palate to the epiglottis and is posterior to the oral
cavity. The opening from the oral cavity to the oropharynx is
called the oropharyngeal isthmus (Isthmus of Fauces). A
difference between the nasopharynx and oropharynx is the
epithelial covering of the passages. In comparison to the
nasopharynx, which is lined with columnar epithelia, the
oropharynx is covered by stratified squamous epithelia, that are
often found covering areas which are subject to a great deal of
frictional wear. The many layers of epithelial cells serve to
protect the underlying tissues from being damaged by the food
and material coming into the passage from the mouth.
-
Laryngopharynx
The third portion of the pharynx is the laryngopharynx. The
shortest of the three parts of the pharynx, it runs inferiorly
from epiglottis and ends superior to the esophagus. The
laryngopharynx carries both air and food and is lined with
stratified sqaumous epithelia.
Tonsils
The paired pharyngeal tonsils, also known as the adenoids, lie in the
posterior wall of the nasopharynx. The other tonsils, the tubal tonsils,
protect the auditory tubes and middle ear against entering
microorganism. They are located at the opening of the tube that connects
the middle ear to the nasopharynx. The auditory or Eustachian tube
equilibrates air pressures between the environment and the middle ear.
The orientation of the auditory tube changes during infancy and early
childhood. The tube is oriented horizontally until around age two.
Consequently, it frequently serves as a point of entry for bacteria in
children less than two years of age. Around age two, the auditory tube
changes course and slants down from the ear, allowing fluid to drain
more readily. Without the fluid to act as a growth medium and conduit to
the middle ear, older children usually experience few ear infections.
The paired palatine tonsils lie along the two sides of the Isthmus of
Fauces in the oropharynx. The lingual (lingual meaning tongue) tonsils
are not in the oropharynx, but are located at the base of the tongue.
Collectively, the tonsils aid in filtering foreign material that could
do harm.
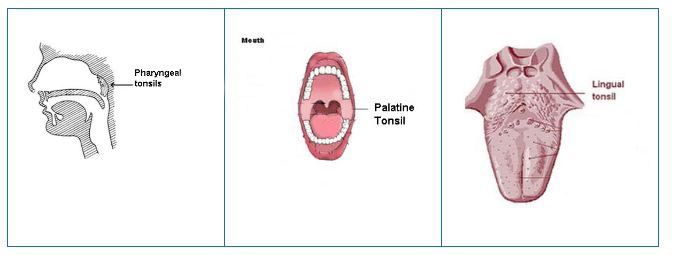 Tonsils.
Tonsils.
Larynx
After air leaves the pharynx, it enters the larynx, a complex structure
that extends from the laryngopharynx and the hyoid bone to the trachea.
It is about 5 cm (2 in.) long. In addition to providing a passageway for
air, the larynx directs air and food to their appropriate tubes. The
airway is blocked by closing off the opening of the trachea with the
epiglottis upon swallowing. The vocal cords, which are used in making
sound and speech, can be found within the larynx. The epithelial lining
of the larynx exhibits two different arrangements. Initially stratified
squamous epithelia lines from the laryngopharynx to the vocal cords.
Inferior to the vocal cords the epithelial lining shifts to
pseudostratified, ciliated, columnar epithelia. The nine cartilage
structures found in the larynx provide key anatomical landmarks. They
function to maintain an open airway. Eight of the cartilages are
composed of hyaline cartilage.
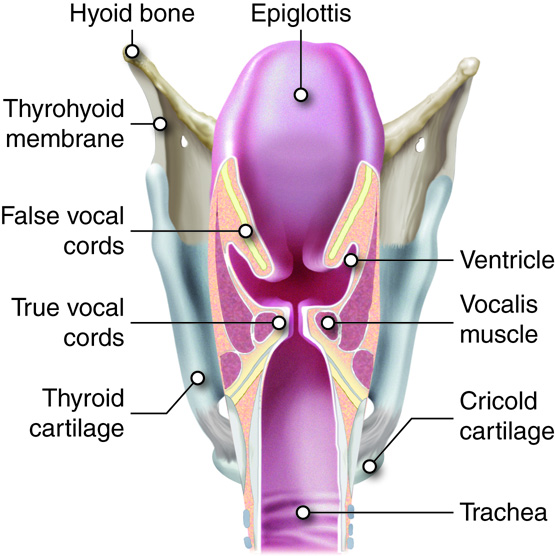 Detail of Larynx. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Detail of Larynx. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
-
Epiglottis
The epiglottis is made of elastic cartilage and covered with
stratified squamous epithelia. It connects loosely to the
tongue, the hyoid bone, and the rim of the thyroid cartilage.
The epiglottis is normally open, allowing air to freely flow
into the rest of the larynx and the trachea. When a person
swallows, the front of the epiglottis is raised, and the
posterior portion descends, covering the glottis, which is the
opening to the vocal cords and trachea. This movement directs
food and water to the esophagus and prevents it from entering
the bottom portion of the larynx and the upper trachea. If
someone tries to talk or laugh and swallow at the same time, the
air used to talk or laugh forces the epiglottis open, the
swallowed material “goes down the wrong tube,” and choking
results.
-
Thyroid cartilage
The thyroid cartilage is the largest of the cartilages of the
larynx and is found at the front of the larynx. In the fetus the
cartilage originates as two separate plates that fuse before
birth. This roughly triangularly shaped cartilage contains the
laryngeal prominence, commonly known as the “Adam’s apple”. The
laryngeal prominence becomes more prominent in males during
puberty as the larynx widens and the voice deepens. The thyroid
cartilage connects to the hyoid bone by the thyrohyoid membrane
or ligament.
-
Cricoid cartilage
Inferior to the thyroid cartilage is the cricoid cartilage. It
connects superiorly to the thyroid cartilage by the cricothyroid
ligament and inferiorly to the trachea by the cricotracheal
ligament. When an occlusion of the upper respiratory tract
occurs and a tracheostomy is performed to facilitate breathing,
the cricothyroid ligament must be punctured.
-
Arytenoids cartilages, cuneiform cartilages,
corniculate cartilages
The next six cartilages are found in three pairs. The first
pair, the arytenoids cartilages, anchor the true vocal cords.
The second pair are the cuneiform cartilages, and the third pair
are the corniculate cartilages. All three pairs of cartilages
are found in the lateral and posterior walls of the larynx.
Except for the epiglottis, the arrangement of the cartilages of
the larynx ensure that the passages through the larynx remain
open.
-
Vocal cords
Two pairs of folded tissue can be observed in the larynx
immediately inferior to the epiglottis. These are the false and
true vocal cords respectively. The false vocal
cords do not function in making sounds or speech but aid in
closing the glottis, which opens to the rest of the larynx and
the respiratory system. Below the false vocal cords are the true
vocal cords. The true cords run from the arytenoids to the
thyroid cartilage. They are reinforced with elastic fibers and
vibrate when adequate air is forced through the gap between
them, resulting in sound. Pitch control is achieved by adjusting
the tension on the cords. Lessening the tension lowers the
pitch. During puberty in males the cords are usually lengthened
making for a deeper sound. Actual speech is achieved with the
coordination of muscles in the pharynx, face, tongue, soft
palate, and lips.
Lower Respiratory Tract
Trachea
The flow of air continues from the larynx to the trachea or windpipe. The
trachea is about 10 cm (4 in.) long and 2 cm (3/4 in.) wide and extends
from the larynx to the level of the fifth thoracic vertebrae. The
presence of 16 to 20 C-shaped pieces of hyaline cartilage located along
the trachea prevent this airway from closing. The last cartilage in the
trachea exhibits a projection from the anterior surface that extends
into the lumen. This projection, the carina, is very sensitive to
particulate material and causes coughing when stimulated. The openings
of the C-shaped cartilages face the posterior of the trachea and contain
the trachealis smooth muscle. This muscle constricts when someone
coughs, increasing the force of the cough. The trachealis muscle also
constricts during an asthmatic reaction, shrinking the airway and making
it harder to breathe.
Structurally the trachea consists of three concentric tissue layers: the
mucosa, submucosa, and adventitia. This arrangement is continuous
throughout the remainder of the upper respiratory system. The layer
facing the lumen, the mucosa, is an epithelial layer. The cell types
found in the trachea are pseudostratified, ciliated, columnar epithelia.
In addition prominent elastic fibers can be found in the lamina propria.
The submucosa contains blood vessels, nerves, assorted connective
tissues, and mucus glands. The outer adventitia is mostly areolar
connective tissue that is continuous with the hyaline cartilage and
connects the trachea to the surrounding structures and tissues.
The Bronchi and Bronchioles
The bronchial tree is so named because it resembles a tree that has been
turned upside-down. The trachea would be the trunk, the progressively
smaller bronchi and bronchioles are the branches, and the alveoli are
the leaves. Branching of the trachea into the right and left primary
bronchi occurs after the last cartilage in the trachea at the level of
the seventh thoracic vertebrae. The right primary bronchus is wider,
shorter, and more vertical than the left primary bronchus. The primary
bronchi branch to form the secondary or lobar bronchi. There are three
secondary bronchi on the right and two on the left. The secondary
bronchi continue to branch into the tertiary or segmented bronchi, which
also continue the branching pattern. Approximately 23 successive
branches lead to the bronchioles. A bronchiole is a tube with a diameter
of less than 1 millimeter (mm). When the measurement reaches less than
0.5 mm, a bronchiole is termed a terminal bronchiole.
The bronchial tree is lined by pseudostratified, ciliated, columnar
epithelia with goblet cells dispersed among the columnar cells. Plates
of cartilage are found in the walls of the bronchial tree, with the
amount of cartilage and the number of plates decreasing as the bronchi
and bronchioles become smaller. The terminal bronchioles do not contain
cartilage plates, these tubes are small enough to stay open without
cartilage. Smooth muscle is found throughout the system, even into the
respiratory zone.
The Lungs
The lungs are two of the largest organs of the body, but they are among
the lightest. Along with the heart, the lungs take up nearly all of the
space in the thorax, superior to the diaphragm. As an organ, the lung is
made up of airway tubes and alveoli, giving it little weight. Elastic
connective tissues in the stroma of the lungs allow them to expand with
incoming air and recoil when expelling air. The lungs contain a large
amount of surface area in order to efficiently support the exchange of
oxygen and carbon dioxide.
The hilus (meaning depression or pit) of the lungs is an
indentation on the medial side of the lungs and the point of entry of
blood vessels, primary bronchi, nerves, and lymphatics. This collection
of vessels and nerves makes up the root of the lung. The tip or apex of
the lungs is a blunted point found just above the clavicles. The
posterior, lateral, and anterior sides of the lungs are surrounded by
the ribs. These areas are called the costal surfaces of the lungs
referring to the costal cartilage surrounding them. The flat, inferior
surface of the lungs is found superior to the diaphragm and referred to
as the base of the lung. Since, the liver is found on the right side of
the body and inferior to the diaphragm, the insertion of the diaphragm
is slightly raised on the right. Consequently the right lung is usually
slightly shorter than the left. The lungs extend from the first costal
cartilage to the tenth thoracic vertebrae.
The lungs consist of a right lung and a left lung. Even though the right
lung is slightly shorter than the left, the left lung has about 10
percent less mass than the right due to the cardiac notch on the medial
side of the left lung. The heart is tucked into this notch. The heart,
the right lung, and the left lung, are each located in their own
anatomical compartment in the upper thorax. The right lung is divided
into three lobes. A horizontal fissure separates the superior and middle
lobes, and an oblique fissure separates the middle and inferior lobes.
The smaller left lung contains only two lobes. An oblique fissure
separates the superior and inferior lobes on this side.
Each lobe is divided into bronchopulmonary segments separated by
connective tissue septa. There are a total of 10 of these segments and
each contains a tertiary bronchiole, a pulmonary and bronchial artery,
and a lymphatic branch. The presence of these segments aids in further
isolating parts of the lungs to prevent the spread of infection or
disease. Connective tissue further divides the segments into lung
lobules, the smallest anatomical unit in the lungs. A lobule is
hexagonal in shape and less than a centimeter in diameter. Each lobule
contains a terminal bronchiole and its associated alveoli. The
connective tissue associated with lobules may be blackened by tobacco
smoke or pollution from the environment.
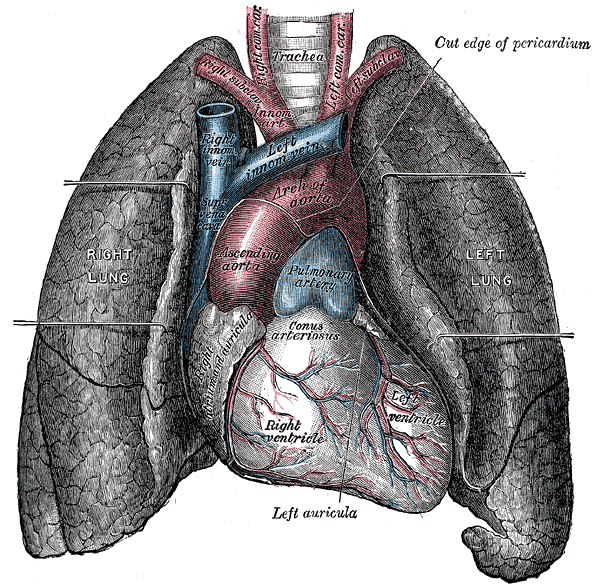 By Gray's Anatomy (Heart and Lungs)
By Gray's Anatomy (Heart and Lungs)
Image below compares the lungs from a smoker with those from a
non-smoker.
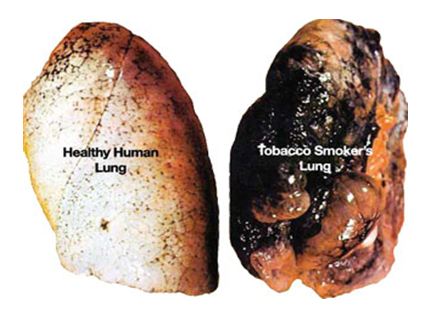 Lungs.
Lungs.
Usually, lungs are a pink with a relatively smooth surface. The lungs on
the right are blackened and irregularly shaped from the accumulation of
material inhaled with tobacco smoke. This material can be cleared from
the lungs, but only over a long period of time and only if the person
stops smoking. Cigarette smoking causes the deaths of over 440,000
people in the United States each year, including people exposed to
second-hand smoke. The financial losses associated with these deaths are
in excess of 200 billion dollars a year.
There are over 4,000 chemical compounds that are created when the more
than 600 chemical ingredients in cigarettes are burned and inhaled.
More than 50 of the chemicals produced by the burning of a cigarette are
classified as carcinogens. A partial list of these chemicals includes:
acetone, acetic acid, ammonia, arsenic, benzene, butane, cadmium, carbon
monoxide, formaldehyde, lead, naphthalene, methanol, nicotine, tar, and
toluene.
The direct effect of many of the chemicals in the smoke is to paralyze
and destroy cilia. When the cilia cannot function, mucus accumulates in
the lung tubules. Opportunistic bacterial and other microorganisms
utilize the mucus to grow and colonize the lungs. Many of these
organisms create pathologic conditions inside the lung.
Smoker’s cough is a condition that exists because the mucus and other
debris accumulate in the lung tissue. These accumulations stimulate the
cough reflex as means to clear the lungs.
The inhaled chemicals can also contribute to the breakdown of connective
tissue fibers, especially elastin. The degeneration of the connective
tissue can lead to emphysema, a form of chronic obstructive pulmonary
disease (COPD).
Blood and nerve supply
The lungs have a dual blood supply. The pulmonary artery brings
oxygen-poor blood from the right ventricle of the heart. This blood
passes through the pulmonary capillaries, where some carbon dioxide will
leave the blood and a large amount of oxygen will be acquired. The newly
oxygenated blood enters the pulmonary veins and returns back to the left
side of heart. The pulmonary circulation holds about 500 milliliters
(ml) of blood, or about 10 percent of the body’s supply. About 75 ml of
blood is in the pulmonary capillaries for gas exchange at any one time.
The blood supply that nourishes the tissues of the lungs arrives through
the bronchial artery, which branches off of the aorta and carries
oxygen-rich blood to support the lung tissues. The bronchial supply
anastomoses with the pulmonary vessels, and a mixture of blood leaves
through the bronchial and pulmonary veins. Blood passes through the
lungs at a rate equal to cardiac output, or about five liters per
minute.
Blood pressure measured in the pulmonary circulation is less than it is
in corresponding vessels of the systemic circulation. To some extent
this decrease results from the decreased resistance found in this
shorter pulmonary pathway. The mean pulmonary arterial pressure of about
15 mmHg is adequate to push blood through the pulmonary capillary
network and into the left side of the heart. The low hydrostatic
pulmonary capillary pressure of 7-9 mmHg only produces a small amount of
fluid filtration across the capillary wall. Under normal conditions,
this filtrate is readily removed by the lymphatic vessels. Under
conditions where left atrial pressure rises dramatically, such as in
mitral valve stenosis or congestive heart failure, pulmonary capillary
pressure will also rise, increasing the rate of capillary filtration. If
the lymphatic system cannot keep up with the higher filtration rate,
fluid will accumulate in the alveoli as pulmonary edema. This can
interfere with the exchange of oxygen, leading to cyanosis, decreased
activity tolerance, etc.
The pulmonary circulation responds to hypoxia differently than the
systemic circulation. The systemic circulation dilates under hypoxic
conditions causing an increased blood flow through the tissues. The
arterioles of pulmonary circulation constrict selectively in cases of
alveolar hypoxia. This constriction diverts blood to areas in the lungs
that are better ventilated. This helps ensure adequate gas exchange.
Nerves from the pulmonary plexus enter the lungs at the hilus. These
nerves contain a mixture of visceral sensory and autonomic nerve fibers
that follow the bronchial tree and blood vessels. Parasympathetic nerve
stimulation results in bronchoconstriction, constriction of the
bronchioles, while sympathetic nerve stimulation results in
bronchodilation, dilation of the bronchioles.
Pleura
Each lung is found in a pleural cavity bounded by the pleural membrane, a
double sided membrane that contains a thin layer of pleural
fluid. The visceral pleural is a mucus membrane that covers
the lungs and folds over at the hilus. The folded membrane continues and
becomes the parietal pleura, which lines the inner wall of the thoracic
cavity. The space between the two membranes is called the pleural cavity
or space. From 1 to 15 ml of pleural fluid is found on the facing
surfaces of the pleural membranes. This fluid helps to lubricate the
membrane surfaces so that the movement of the lungs during inhaling and
exhaling does not cause frictional damage to the tissues. The fluid also
lightly holds the two membranes together so that they move together as
the chest wall expands and contracts.
Since pleural fluid is mainly a filtrate of plasma, the factors that
affect the amount of pleural fluid production and removal are the same
factors that govern interstitial fluid volumes in most regions of the
body. The main factors include capillary hydrostatic pressure, capillary
colloid osmotic pressure, the permeability (“leakiness”) of the
capillaries, and the rate of fluid removal by the lymphatics. Under
normal conditions, there is a slow but steady turnover of pleural fluid,
but under certain conditions excess fluid can build up, and impede lung
expansion. This condition is known as a pleural effusion and may be
secondary to blockade of venous drainage by tumors, increased capillary
permeability because of infections, etc.
The pleural sac extends below the lungs, to the level of the twelfth
thoracic vertebrae. Samples of the pleural fluid can be safely taken
from this area. Normal pleural fluid is clear and pale yellow in color.
It has very few cells free in the fluid. The majority (75 percent) of
these cells are macrophages. About 23 percent of the cells are
lymphocytes, with an assortment of cells making up the remaining 2
percent.
 Lungs, pleural sac. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States(http://creativecommons.org/licenses/by/3.0/us/).
Lungs, pleural sac. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States(http://creativecommons.org/licenses/by/3.0/us/).
-
Pleural Transudates vs. Exudates
Since a pleural effusion may be caused by a number of different
conditions, inspection of the pleural fluid is often be used to
help determine the cause of the effusion. The fluid can either
be classified as a transudate or an exudate, depending on if the
protein content is significantly increased (exudates) or not
(transudate). A transudate forms when the forces contributing to
capillary exchange are altered, such as with venous blockade
that increases hydrostatic pressure, or when lymphatic drainage
is impeded. An exudate occurs when the permeability of the
capillaries increases, such as with infections or localized
cancers.
Gross examination of pleural fluids can help to distinguish
between transudates and exudates. Both conditions produce an
increase in fluid volume. Transudates will appear similar to
normal pleural fluid. It will appear clear and slightly yellow
in color. Exudates will be cloudy and gray in appearance.
Transudates will also have a normal cell count and distribution.
Exudates will have an increased total cell count. For example,
an increase in lymphocytes occurs in tuberculosis, and an
increase in neutrophils occurs in bacterial infections.
Respiratory Function
The movement of gases from the atmosphere into the lungs and the cells of the
body and back out again is called external respiration. External
respiration includes ventilation, the bulk flow of air from outside the body
into the repiratory system (inhalation) and the bulk flow of air from the
respiratory system back to the outside (exhalation). Also included under the
processes described as external respiration are the exchange of oxygen and
carbon dioxide between the lungs and the blood, the transport of oxygen and
carbon dioxide by the blood, and the exchange of gasses between the cells and
the blood. The conduit for moving air into the lungs and then returning it to
the external environment is referred to as the conducting zone
(division) Gas exchange does not take place in the structures that make up the
conductiong zone. The conducting zone consists of a system of tubes that connect
the nose and mouth to the alveoli. Air movement starts at the nose or mouth and
continues through the pharynx, larynx, trachea, and bronchial tree, terminating
at the structures of the respiratory zone (division) where gas
exchange will occur.
The respiratory zone includes the very small respiratory bronchioles, the alveoli
and the blood capillaries. It is critical that both the conducting and
respiratory zones function adequately for the body to maintain homeostasis.
Organization of conducting and respiratory zones will be the focus of this
discussion.
The Conducting Zone
The conducting zone of the respiratory system brings gases into and out
of the respiratory system. In addition, the organs and tissues along
this path warm the incoming air to approximately body temperature,
moisten the air to about 100 percent humidity, and begin filtering out
any harmful microorganisms or particles that may be suspended in the
inhaled air.
Temperatures too far below 37°C (98.6°F) can harm the delicate alveoli
and inhibit enzyme function. Damaged aveoli, or improperly functioning
enzymes could lead to decreased gas diffusion and subsequent homeostatic
imbalances. For example, the inter-conversion of carbon dioxide and
carbonic acid is catalyzed by the enzyme carbonic anhydrase. Similar to
most other body enzymes, it loses efficiency at lower temperatures. If
carbonic anhydrase is not functioning properly there could be an
increase in carbonic acid levels. Since carbonic acid breaks down into
bicarbonate ions and free hydrogen ions, there would be resulting
decrease in pH leading to acidosis. The temperature in the respiratory
zone is so important that a person who runs in cold weather is advised
to put a scarf across their nose and mouth to help pre-warm the air.
Otherwise, the tissues in the conducting zone may not be able to warm
the air sufficiently to prevent damage to the system.
If the air in the respiratory zone is not near 100 percent humidity, the
thin-walled alveoli can become dehydrated and may deteriorate. The
alveoli are lined with a very thin layer of water and chemicals that
prevent damage to the tissues and help to keep the alveoli open. If the
air taken to the respiratory zone doesn’t have sufficient humidity
(water vapor), the water layer lining the aveoli will evaporate
resulting in damage to the structures and interference with
respiration.
The hairs found at the entrance to the nose, along with the mucus and
cilia found inside the nose help to trap and remove pathogens and
particles taken in with air. Large particles can build up at the
entrance of the nose where the hairs trap them. Coal miners, for
example, will accumulate coal dust at the opening of their nostrils due
to the action of these hairs. Smaller particles, such as some viruses
and bacteria will be trapped in the mucus in the nose and the cilia on
the epithelial cells will sweep them to the back of the pharynx. The
combined action of the mucus and the cilia is referred to as the
mucociliary escalator. Ultimately the mixture of mucus and particles
will be swallowed and destroyed by the acid and digestive enzymes in the
stomach. This process of trapping and removal of particles and
microorganisms occurs throughout the conducting zone and through the
first part of the respiratory zone.
The Respiratory Zone
A transition occurs in the composition of the epithelial lining at the
end of the terminal bronchioles. The epithelial lining changes to simple
cuboidal cells without any intermixed goblet cells. The walls of the
tubes at this point become very thin and are called respiratory
bronchioles. The loss of the goblet cells means that no mucus is
secreted into the bronchioles at this point, but the cilia are still
present to sweep away any mucus that should come down into them. Some
portions of the respiratory bronchioles are not completely covered by
cilia, and a number of phagocytic macrophages are found in this area to
compensate for the loss of the cilia. Some gas exchange can occur
through the walls of the respiratory bronchioles.
Respiratory bronchioles lead into the alveolar ducts, which still have
smooth muscle and some elastic fibers in the walls. Alveolar sacs occur
as clusters of aveoli (singular alveolus) sharing a common chamber along
the alveolar ducts. It has been estimated that there are 300 million
alveoli in the lungs. These alveoli provide a surface area of 27 square
meters, or an area 25 feet by 30 feet, which is roughly the size of a
three-car garage. Adjacent alveoli are interconnected through alveolar
pores.
Pulmonary Ventilation
Pulmonary ventilation is the act of breathing and the first step in
the respiratory process. Pulmonary ventilation brings in air with a new supply
of oxygen and a very small amount of carbon dioxide from the atmosphere into the
alveoli. This mixture then participates in external respiration,
the exchange of oxygen and carbon dioxide between the alveoli and pulmonary
capillary blood across the respiratory membrane. Internal respiration
is the exchange of gasses between the tissues of the body and the blood,
which provides oxygen for aerobic cellular respiration and removes carbon
dioxide. Aerobic Cellular respiration refers to the intracellular
use of oxygen and the generation of carbon dioxide waste through metabolic
pathways.
Pressure
Breathing or ventilation, involves changes in pressure as a result of
mechanical work.
The physical movement of air into the lungs is a result of the
production of differences in total pressure between the interior
(alveolar pressure) and exterior (atmospheric pressure) of the
respiratory zone. Atmospheric pressure at sea level is typically 1
atmosphere or 760 mmHg, and this value will be used as the reference
atmospheric pressure here.
One atmosphere is the air pressure that would push a column of the
mercury up in a thin tube a distance of 760 mm. Mercury is used because
it is a liquid at room temperature, and its density changes very little
over pressure and temperature ranges. In the process of ventilation, air
moves down pressure gradients going from an area of higher pressure to
an area of lower pressure. Assuming a person is at sea level, if their
intrapulmonary pressure, the pressure in the alveoli, is less than 1
atm, air enters the lungs and fills the alveoli. If their intrapulmonary
pressure is greater than 1 atm, then air moves out of the lungs into the
environment.
There is another pressure often discussed in the mechanics of
ventilation, intrapleural (sometimes just pleural) pressure. This is the
pressure within the pleural sac (space). Intrapleural pressure is
maintained slightly less than the pressure in the alveoli. This pressure
difference aids in keeping the lungs slightly inflated at all times and
ensures that they do not collapse when exhaling.
 Close up intercostal muscle. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Close up intercostal muscle. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Inspiration and Expiration
Gases have a property of distributing to fill whatever size and shape
container they occupy. If a closed container is made larger (an increase
in volume), the total number of gas molecules will stay the same, but
they will redistribute to fill the larger space. In doing so, they
decrease their concentration (remember that concentration is a property
of mass and volume). This increase in volume but not number of molecules
of the gas leads to a corresponding decrease in the pressure exerted by
that gas on the walls of the container. If the same closed container is
made smaller, the concentration of gases increases( even though the
actual number of gas molecules has not changed) and the pressure
increases. This is an example of a physical law called Boyle’s
Law.
The law is expressed mathematically as:
where P = pressure, V = volume, 1 is the initial pressure and volume, and
2 is the resulting pressure and volume.
Boyle’s Law states that, at a constant temperature, the pressure of a gas
is inversely proportional with the volume of its container. Very simply,
as volume goes up, pressure goes down, and as volume goes down, pressure
goes up. These properties become important for ventilation because the
passage of air into and out of the lungs is controlled by the size of
the lungs’ container, the thorax. The difference between the example
above and the lungs is that the lungs are not a closed system (unless
you have a closed glottis), with the opening to the lungs maintained
through the conducting zone. But over very short periods of time Boyle’s
law still applies. In this case, when we inhale, the movement of the
diaphragm and the ribs increases the volume of the thorax. This
immediately decreases the air pressure within the thorax, creating a
pressure gradient between the atmosphere and the alveoli (the latter is
called intrapulmonary pressure), and drawing air into the lungs. Air
will enter until the atmospheric and intrapulmonary pressures are equal.
However, the volume of the thorax remains larger than before the start
of inhalation. To exhale, the thorax is allowed to decrease in volume
(the ribs and diaphragm return to their original positions), increasing
the intrapulmonary pressure and creating a gradient in the opposite
direction resulting in the flow of air out to the atmosphere. Changing
the size of the thoracic cavity would be impossible if the ribs were
solidly attached to the sternum. If they were solidly attached, it would
be like trying to move the sides of a birdcage. The costal cartilage
allows the ribs to move and expand or contract the chest wall.
Inspiration
Inspiration is the act of inhaling. As stated above, inspiration
occurs by increasing the volume of the thorax. This active
process involves the use of chest and neck muscles. Resting
inspiration is achieved mostly by movement of the diaphragm. The
relaxed shape of the diaphragm resembles a shallow dome with the
apex pointing toward the lungs, similar to the shape of an open
umbrella. When the diaphragm contracts, it tends to flatten out,
expanding the volume of the thorax in an inferior direction.
Consequently, the intrapleural and intrapulmonary pressures
decrease below atmospheric pressure resulting in air being
pulled through the conducting zone and into the lungs. The
external intercostal muscles work in conjunction with the
diaphragm. The normal orientation of the ribs is around the side
of the thorax and angled inferiorly to the sternum. When the
external intercostal muscles contract, the rib are pulled up,
also expanding the thoracic cavity in a horizontal direction. In
adults, ventilation occurs about 12 times a minute and moves
roughly 500 ml of air during each breath.
A deep breath involves a greater shortening of the diaphragm and
the external intercostal muscles plus additional contractions of
other neck and chest muscles. The scalene muscles elevate the
first two ribs. The sternocleidomastoid muscles elevate the
sternum, and the pectoralis minor muscles help to elevate the
third, fourth, and fifth ribs. Two physical conditions that
interfere with inspiration are obesity and advanced pregnancy.
In both cases, the abdominal organs are pushed against the
diaphragm, restricting its downward movement and hindering the
expansion of the thoracic cavity.
Expiration
Expiration is the act of exhaling. Resting expiration is a
passive process. The muscles used during inspiration relax, and
allow the chest wall and the diaphragm to move back to their
original position, thus decreasing the volume of the thorax and
forcing air from the lungs. The compression of the chest wall
also aids in moving blood and lymph through the vessels that
drain the lungs. Expiration becomes an active process when a
more forceful exhale is required. The internal intercostal
muscles pull the ribs down, helping to compress the chest. The
external and internal oblique and transverse abdominal muscles
press on the abdominal organs, which move them up against the
diaphragm and force the diaphragm higher than it would normally
go on relaxation, further decreasing the volume of the thoracic
cavity.
Airway resistance, alveolar surface tension, and lung compliance
One thing that works against the pressure gradient created by the
expansion of the thorax is the resistance found within the conducting
zone. Under normal conditions this resistance is quite low, and the
airways have little trouble passing air between the atmosphere and the
alveoli. The small amount of resistance found in the system stems mainly
from the bronchioles, which are analogous to the arterioles of the
vascular system. Both the bronchioles and the arterioles contribute a
large portion of the resistance of their entire system, and both have
smooth muscles in their walls that allow them to constrict or dilate.
Under conditions of bronchiolar constriction (bronchoconstriction),
resistance to airflow can increase dramatically. When this happens
rapidly, it is classified as an acute asthma attack. The afflicted
individual will need to generate much larger changes in intrapulmonary
pressure to maintain a normal flow rate of air during breathing. Recall
that if resistance increases an increased pressure gradient is necessary
to maintain the same flow. To achieve this, the person uses the accessory
muscles to breathe, and will appear to be “straining” as they do so.
Treatment usually involves an inhaler containing a bronchodilator.
Resistance will also be affected if the airways become narrowed by mucus
(chronic asthma) or aspirated substances.
Along with the resistance of the airways, the compliance of the lung
tissue also determines how much effort breathing requires. Compliance is
a property that explains the relationship between a volume change and
pressure change. Typically it takes a 2-3 cm of water change in pleural
pressure to change volume in the lungs by 500 ml. This is a compliance
of about 200 ml/cm H2O. The compliance of the lungs is
dependent on the elasticity of the connective tissues of the lung as
well as the alveolar surface tension. Remember that a thin liquid layer
containing water as well as other molecules lines the interior lung
surface. Because of its polar nature, water exhibits cohesion, such that
it takes some effort to separate water molecules (you may have
experienced the force of this cohesion if you ever belly flopped into
the water). This surface tension is actually enough to collapse the
alveoli each time a person exhales. In order to prevent this from
happening, the type II alveolar cells make a combination of lipids and
proteins that serves as a surfactant in the alveoli. A surfactant is a
chemical that acts like a detergent breaking the surface tension of the water
lining the alveoli. This action allows the alveoli to remain open when exhaling.
Compliance of the lung can be decreased either by fibrosis of the lung
tissue creating a stiffening of the tissue (this can occur with
conditions such as tuberculosis) or lack of surfactant (common in
premature infants). Either way breathing becomes much more difficult,
requiring more effort and sometimes mechanical ventilation.
Various lung volumes, capacities (capacities are combinations of lung volumes)
and flow rates can be evaluated through a process called spirometry. The patient
breathes in and out under controlled conditions, and the amount of air passing
through the system, as well as the time it takes for air passage is measured.
The values for these measurements depend on lung function and the size of the
patient.
| Respiratory Volumes |
Description |
Normal adult values |
| Tidal Volume (TV) |
Volume of a resting breath |
500 mL/breath |
| Inspiratory Reserve Volume (IRV) |
Maximum volume that can be inhaled after a normal inhale |
1900 – 3300 mL |
| Expiratory Reserve Volume (ERV) |
Maximum volume that can be exhaled after a normal exhale |
700 – 1200 mL |
| Residual Volume (RV) |
Air left in the lungs after exhaling completely (i.e. after an ERV) |
1200 mL |
| Dead Space |
Air inhaled during breathing that stays in the conducting zone |
150 mL |
A normal, resting respiration is called the tidal volume (TV), and
is typically about 500 ml per breath. Inspiratory reserve volume (IRV)
is the amount of air that can be taken in “on top of” the tidal volume,
with a deep breath. This ranges from 2,100 to 3,200 ml. The reverse of the IRV
is the expiratory reserve volume (ERV), the amount of air that can
be forcefully expelled after the tidal volume. The ERV range is from 1,000 to
1,200 ml. The conducting zone organs contain a volume of air that does not enter
the respiratory zone (and therefore does not participate in gas exchange) called
the dead space air. The amount of dead space air is typically 150 ml. The actual
air will be moved during the next inspiration or expiration, but the volume of
dead space air does not enter into the volumes involved in internal
respiration.
The final normal respiratory volume is the residual volume (RV).
This is the amount that is left in the lungs after the ERV is expelled. The RV
is about 1,200 ml of air. The residual volume helps maintain open alveoli and is
the air that the newly inhaled air is mixed with. No matter how hard a person
exhales, there is always air left in the lungs. This remains true even after
death. The only way to remove the residual air from the lungs is to replace it
with something else.
Essentially this is what happens when a person drowns, water replaces residual
air. If a pathologist needs to determine if a deceased person recovered from a
body of water drowned or was dead before going into the water, he or she clamps
off the trachea, removes the lungs, and places them in a bucket of water. If the
person drowned, the lungs will sink because the residual air was replaced by the
water. If the person was dead before going into the water, the lungs will float
because the residual air is still there.
learn by doing
Spirometry is the measuring of breath and is one of the most common tests
of pulmonary function. These tests are collectively known as PFTs
(pulmonary function tests) and are used to measure and assess the
ventilation of how the lungs. Spirometry can measure the volume of air
moving in and out of lungs and the speed of airflow in and out of the
lungs. The picture below is an example of a spirogram that is showing a
volume vs time curve. The most common features measured in spirometry
are the vital capacity, the forced vital capacity, and forced expiratory
volume.
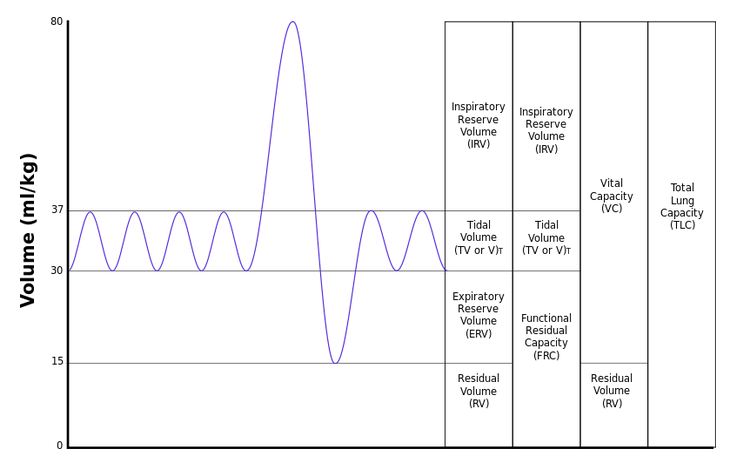 By Vihsadas at en.wikipedia derivative work (Lung Volumes) CC-BY-SA-3.0
By Vihsadas at en.wikipedia derivative work (Lung Volumes) CC-BY-SA-3.0
Several respiratory capacities can be calculated from the volumes listed above.
The inspiratory capacity (IC) is the total amount of air that can
be inhaled and is calculated by adding the tidal volume and inspiratory reserve
volume. The functional residual capacity (FRC) is the amount of air
remaining in the lungs at the end of a normal exhalation. This air continues to
participate in gas exchange even after the person has exhaled. Functional
residual capacity is calculated by adding together residual and expiratory
reserve volumes. The vital capacity (VC) is the total exchangeable
air in the system and is calculated by adding together tidal volume, inspiratory
reserve volume, and expiratory reserve volume. Typically vital capacity is about
4,800ml of air. The total lung capacity (TLC) is the total of all
lung volumes and is about 6,000 ml.
| Respiratory Capacities |
Description |
Normal adult values |
| Inspiratory Capacity (IC) |
TV + IRV |
2400 – 3800 mL |
| Functional Residual Capacity (FRC) |
RV + ERV |
1800 – 2200 mL |
| Vital Capacity (VC) |
TV + IRV + ERV |
3000 – 4600 mL |
| Total Lung Capacity (TLC) |
TV + IRV + ERV + RV |
4200 – 6000 mL |
Besides volumes and capacities, flow rates are often measured to assess a
person’s lung function. Flow rates are important because although changes in airway
resistance will not usually change volumes, they will affect the rate of
air movement through the system. To assess flow rates, the individual takes as deep
a breath as possible (gets to VC) and then exhales maximally and as quickly as possible. The
time it takes to bring the lung volume down to RV provides information about
airway resistance. Flow rates are used to assess asthma and related
conditions.
did I get this
Another common pulmonary function test measured by spirometry is the
speed of airflow in and out of the lungs. The FEV1/FVC ratio is commonly
used in the diagnosis of obstructive and restrictive lung diseases. This
ratio is the forced expiratory volume in one second divided by the
forced vital capacity. In healthy adults this ratio should be between
75-80%. Please examine the chart provided to assist you in answering
the following questions.
 By Mikael Häggström (Lung Volumes)
By Mikael Häggström (Lung Volumes)
Lung volumes and flow rates can be used to differentiate between obstructive and
restrictive pulmonary diseases. Obstructive pulmonary disorders are those that increase
the resistance to airflow, thus increasing the time it takes to move air in and
out of the lungs. Examples of obstructive disorders include bronchitis and
asthma. Restrictive pulmonary disorders are those that affect the compliance of the lung
or affect the ability of the chest wall to expand and relax normally. Such
disorders lead to lower lung volumes than would be expected based upon a
person’s size. Examples of restrictive pulmonary disorders include those that lead to
structural or functional changes in the lung tissue (tuberculosis, fibrosis) or
those that impede normal muscular function (such as muscular dystrophy) or
function of motor nerves (such as multiple sclerosis and ALS).
Let’s summarize
Structure determines function. To understand how the respiratory system performs
its function it is essential to examine the anatomical structures of the system.
Previously we examined the microscopic components of the respiratory system. Now
we will places those components into the gross anatomy of the upper and lower
respiratory tracts. After examining the structure we discussed how these
structures perform the functions of the respiratory system and how the
efficiency of these functions is measured.
- Entrance to the upper respiratory tract is through the nose or the mouth.
- The nasal cavity contains structures such as hair, sebaceous glands
and mucus that aid in preventing microorganisms from entering.
- The nasal cavity also contains olfactory epithelium for the sense of
smell.
- The conchae of the nose contain mucus glands and blood vessels to
aid in humidifying and warming the air.
- The paranasal sinuses are hollow areas that make the skull lighter
and also moisten and warm incoming air.
- Next the air passes into the pharynx which is divided into three areas and
connects to the larynx.
- The nasopharynx is the superior portion of the pharynx and only acts
as a passageway for air.
- The oropharynx extends from the soft palate to the epiglottis, and
is covered in stratified squamous epithelia to protect it from the
friction of food as it is swallowed.
- The laryngopharynx is the third and shortest portion of the pharynx.
It extends from the epiglottis and ends above the esophagus. It is
lined with stratified squamous epithelia to protect it from the
friction of food as it is swallowed. Both the oropharynx and the
laryngopharynx are passageways used to transport both air and
food.
- The tonsils are patches of lymphatic tissue that help protect the body
against pathogens by filtering them from the air.
- Pharyngeal tonsils (adenoids) are located in the nasopharynx
- Tubal tonsils protect the auditory tubes and middle ear.
- Palatine tonsils are located in the oropharynx
- Lingual tonsils are located under the tongue.
- The path of air then continues from the pharynx to the larynx which extends
from hyoid bone to the trachea. At the juncture of the pharynx and the
larynx air and food are directed into the larynx (and then trachea)and
esophagus respectively.
- The glottis or opening to the larynx and thus the passageway to the
trachea is blocked by the epiglottis when swallowing.
- The vocal cords are located in the larynx and used for speech
- The larynx also consists of nine cartilage structures that we use as
anatomical landmarks. They function to maintain an open airway. The
cartilages include the thyroid cartilage sometimes known as the
Adam’s apple, the inferior thyroid cartilage (punctured when a
tracheostomy is performed). The paired arytenoid, cuneiform, and
corniculate cartilages.
- Although the lower respiratory tract begins at the larynx, most of the tract
consists of the trachea, the bronchi and bronchioles, the alveoli and the
lungs.
- The trachea, known as the windpipe, extends from the larynx to the
fifth thoracic vertebrae. It is composed of C-shaped cartilage that
maintain an open airway. The trachea consists of three layers of
tissue the mucosa, submucosa and adventitia.
- The bronchial tree is next and is a series of passageways that grow
smaller and smaller as they extend into the lungs. The cartilage
plates are not present in the bronchioles. Plates of cartilage help
maintain the opening of the passageways. As the size of the
passageway decreases so does the number of plates. The respiratory
bronchioles are the smallest and end in the alveolar sacs where gas
exchange will occur.
- The lungs contain elastic connective tissue that allow them to
expand and recoil during ventilation.
- Anatomical reference points of the lungs include 1) the hilus, the
entry point for blood vessels, the primary bronchi and nerves 2) the
apex of the lung, the most superior portion of the lung, and 3)the
base of the lungs, located above the diaphragm.
- There is a right and a left lung. The right lung consists of three
lobes while the left lung only has two lobes because of the location
of the heart.
- Each lobe is divided into many lobules each of which contains a
terminal bronchiole and its respiratory bronchioles and associated
alveoli.
- The lungs have a dual blood supply. The pulmonary circuit brings
deoxygenated blood to the lungs to release carbon dioxide and pick
up oxygen to return to the heart. The systemic circuit brings
oxygenated blood to feed the tissue of the lungs.
- The lungs are surrounded by the visceral pleural which is a mucus
membrane and then the parietal pleural that attaches to the walls of
the thoracic cavity.
- The space between the membranes is the pleural space and contains
pleural fluid that can be examined for signs of infection.
- Pleural effusion can be caused by different conditions so the fluid
is checked to see if it is exudate with increased proteins or
transudate with normal protein levels.
- The primary function of the respiratory system is the movement of gases from
the atmosphere into the lungs and to provide oxygen to the body while
ridding the body of carbon dioxide.
- External respiration includes ventilation and the movement of gasses
between the lungs and the blood and between the blood and the
systemic tissues.
- The passageway for air moving into and out of the body can be
divided functionally into two divisions: the conducting zone and the
respiratory zone which allows for air to diffuse from the lungs into
the blood.
- The conducting zone brings air into and out of the lungs. As the air
passes through the conducting zone it is warmed to body temperature,
humidified and microorganisms are filtered out.
- The temperature and humidity must be correct so the delicate
respiratory zone is not damaged.
- The respiratory zone consists of the respiratory bronchioles and the
alveolar sacs and is the location of gas exchange between the lungs
and the blood.
- Pulmonary ventilation is the act of moving air into and out of the lungs and
is the first step of external respiration.
- Pressure plays a huge role in moving air into and out of the lungs.
Air follows pressure gradients moving from an area of high pressure
to an area of low pressure.
- Air will move into the lungs if the alveolar pressure is less than
the atmospheric pressure and air will move out of the lungs if the
alveolar pressure is higher than the atmospheric pressure.
- Intrapleural pressure is the pressure within the pleural space and
it will help keep the lungs slightly inflated to prevent collapse
during exhalation.
- Pulmonary ventilation includes both inspiration and expiration. To
understand how air moves into and out of the lungs, it is important to
understand the physical laws that govern molecular movement.
- Boyle’s law states that initial pressure times initial volume is
equal to the resulting pressure times the resulting volume.
- This means as volume goes up pressure goes down and as volume goes
down pressure goes up.
- During inhalation the diaphragm and the external intercostal muscles
of the ribs contract and move to increase the volume of the thorax
resulting in a decrease the intrapulmonary pressure and causes air
to move in.
- During exhalation the diaphragm and the external intercostal muscles
of the ribs relax and decrease the volume of the thorax resulting in
an increase in the intrapulmonary pressure and causes air to move
out.
- During forced inspiration contraction of additional muscles of the
neck will allow more air to enter.
- During forced expiration muscles of the abdomen help force the
diaphragm up further decreasing the volume of the thoracic cavity
and increasing the volume of air exhaled.
- Additional factors that affect the pressure gradient include airway
resistance, alveolar surface tension and lung compliance.
- Smooth muscle of the bronchial tubes can constrict or dilate
to alter the airway resistance.
- Conditions such as asthma can cause an increase in
resistance that will result in a person needing to use
accessory muscles to maintain adequate air flow.
- Compliance will also affect the amount of effort required
for breathing. Compliance depends on the elasticity of the
connective tissue in the lungs as well as the alveolar
surface tension.
- Compliance can be decreased by fibrosis of the lung tissue
or by the lack of surfactant.
- To evaluate the efficiency of a persons inhalations and exhalations the
process of spirometry is used.
- A person breathes in and out under controlled conditions and the
amount of air and the flow rate can be measured.
- The respiratory volumes include: Tidal Volume, Inspiratory Reserve
Volume, Expiratory Reserve Volume, Residual Volume, and Dead
Space.
- The Respiratory Capacities are two or more respiratory volumes added
together. They include: Inspiratory Capacity, Functional Residual
Capacity, Vital Capacity, Total Lung Capacity
- Flow rates can also be used to assess a person’s lung function
because there can be changes in the air flow that does not affect
the volumes.
The lungs function to provide the entire body with the oxygen required to convert
nutrients to energy. The lungs also need to be able to remove excess carbon
dioxide from the body. To measure assess a person’s lung function a person can
breathe into a spirometer. The patient’s Respiratory Volumes and Capacities and
flow rate can be measured. These measurements will vary based on a person’s
size, age and gender. Restrictive lung disorders such as tuberculosis and
obstructive disorders such as bronchitis can impede normal airflow and can be
differentiated using spirometry.
Our bodies exchange oxygen for carbon dioxide at a number of different levels. The
exchange of air from the outside environment into the lungs is driven by the mechanics
of ventilation. At a molecular level oxygen binds to hemoglobin in the red blood cells
in the capillaries of the lungs. Some of this oxygen displaces carbon dioxide that was
transported from peripheral cells. The exchange of gasses occurs in red blood cells
(where hemoglobin is concentrated) at the interface of the circulatory system and
respiratory system, called the respiratory membrane. Oxygen
diffuses from the inhaled air in the lungs across the aveolar and capillary membranes
and into the blood plasma. It then enters the red blood cells where it will be carried
on hemoglobin molecules to the other tissues of the body.Gas exchange at the respiratory
membrane is known as external respiration. Gas exchange at between the tissues and the
blood is internal respiration. At the molecular level carbon
dioxide created during cell metabolism diffuses across the cell membrane into the
interstitial spaces and extracellular fluid. When it crosses the capillary and red blood
cell membrane some carbon dioxide is picked up hemoglobin by molecules inside the red blood cell. Inside the cells of the
body tissues, oxygen will be used during the process of aerobic cellular
respiration. Cellular respiration is a process used by our cells to convert
nutrients into energy. The process of aerobic cellular respiration requires oxygen and produces carbon dioxide as a
waste product.
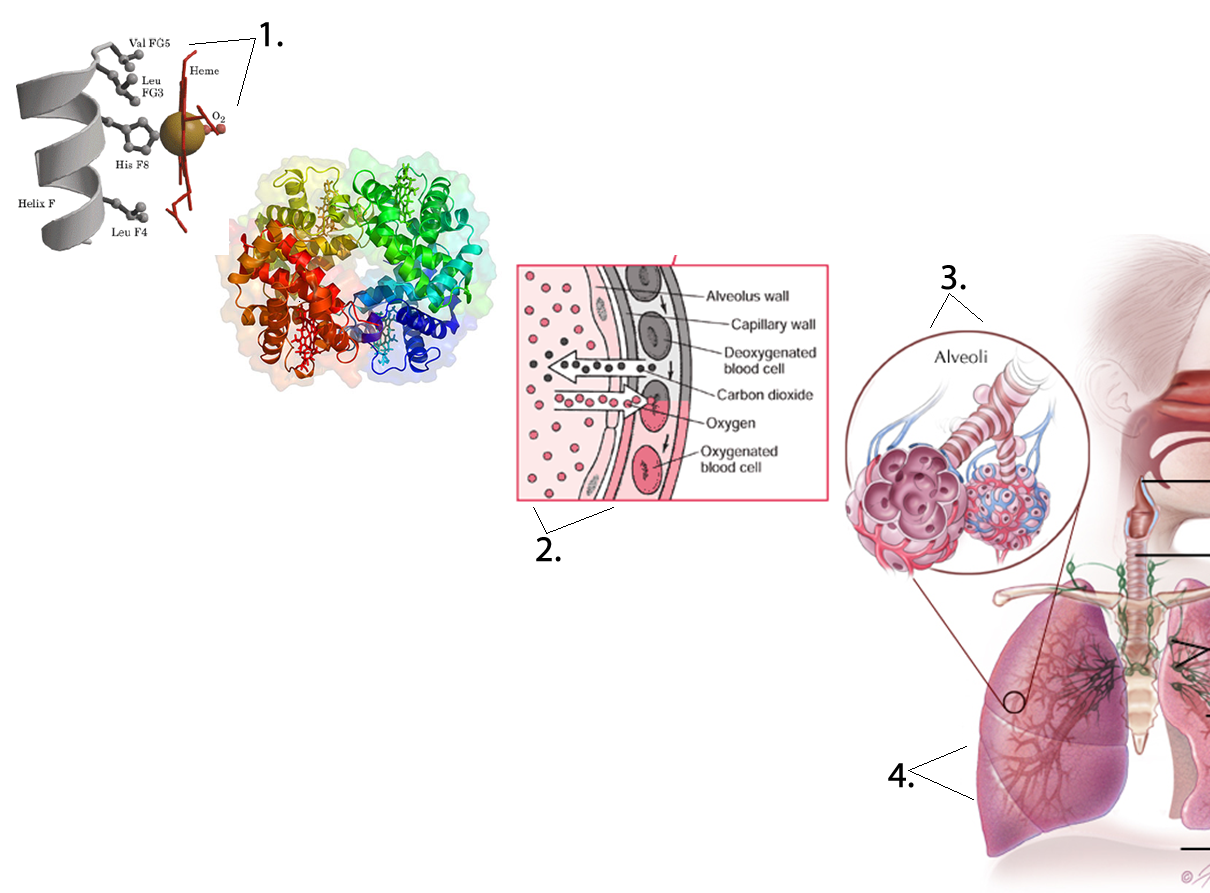 Four respiratory processes. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Four respiratory processes. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
To understand these system-level functions, you will be further exploring structures and
processes occurring at all levels of organization. Some examples of major respiratory
structures assigned to their structural level of organization of the respiratory system
include:
-
Chemical level – oxygen, carbon dioxide, bicarbonate and
hydrogen ions
-
Macromolecular level – hemoglobin, mucus, and surfactant
-
Cellular level – ciliated cells, goblet cells, alveolar
cells, and macrophages
-
Tissue level – stratified to pseudostratified to simple
squamous epithelium
-
Organ level – upper respiratory tract, bronchial tree and
lungs
-
Organ system level - integration of organs for gas
exchange
Air
The four main gases in the atmosphere are nitrogen at 78.084%, oxygen at 20.946%,
argon at 0.934%, and carbon dioxide at 0.039%. Other elements that account for
less than 0.002% of the whole including neon, helium, krypton, hydrogen, nitrous
oxide, carbon monoxide, xenon, ozone, nitrogen oxide, and trace amounts of
ammonia. Water vapor ranges from 1 to 4 percent at sea level and 0.4% overall.
The partial pressure of each of these elements can be found by multiplying the
percentage of the particular gas by 1 atm or 760 mmHg, so the partial pressure
of oxygen (PO2) is 760 x 0.20946, or 159.19 mmHg. The PCO2
is 760 x 0.0039, or 3 mmHg.
learn by doing
The partial pressure of oxygen is what determines how much oxygen is available in
our lungs for transfer to the blood. Atmospheric pressure is 760 mmHg and the
concentration of oxygen is 21% (21% is the same as a fraction of 0.21). Either a
change in the amount of oxygen or a change in the atmospheric pressure can alter
the partial pressure of oxygen (or the amount of oxygen available), but often it
is the pressure that affects how much oxygen is available.
How Gasses Exchange
The respiratory system brings in oxygen and exchanges it for carbon dioxide.
Oxygen makes up 21% of the air we breathe while carbon dioxide is found at very
low levels (0.039%). The diffusion of gasses across membranes follows the same
principles as the diffusion of solutes in solution across membranes, basically
molecules move down a gradient. The difference is that gasses in solution are
measured in terms of partial pressure.
Partial pressure is the pressure that a given gas in a mixture contributes to the
total pressure inside the container or in the atmosphere. The partial pressure
is equal to the total pressure times the fraction of the gas.
For example, at sea level, total atmospheric pressure is 760 mmHg. Because 21% of
air is oxygen, the partial pressure of oxygen in atmospheric air at sea level is
760 mmHg x 21% = 160 mmHg. At higher altitudes, where atmospheric pressure may
only be 500 mmHg, the partial pressure of oxygen would be 500 mmHg x 21% = 105
mmHg.
When we inhale air all the major components of air are in gas form. However, the
oxygen that enters our body must move from the air space in the lungs into the
liquids found in our body such as blood plasma and cell cytoplasm.
Alternatively, the carbon dioxide in the blood moves from the cell cytoplasm and
the plasma in the air of the alveoli. To understand how the air-liquid interface
affects the amount of oxygen or carbon dioxide found in the compartments of the
body, we need to examine Henry’s law, which describes how gasses dissolve in
liquids based on their physical properties and the temperature of the liquid.
Henry’s Law, explains that the amount of dissolved gas found in a liquid is
proportionate to the gas phase partial pressure as well as the molecules
solubility in a specific liquid. For example, considering partial pressures
alone it would be expected that oxygen would readily diffuse from the aveoli
because there is a high partial pressure gradient between alveoli and blood.
However, oxygen has very low solubility in plasma, so even with the large
gradient between the two very little of oxygen leaves the aveoli and dissolves
directly into the plasma. Instead most of the dissolved oxygen found in the
blood is attached to the hemoglobin molecules found inside the red blood cells.
On the other hand carbon dioxide is very soluble in plasma (over 20 times more
soluble in oxygen), so significant amounts can be found directly in dissolved
form. Because of its high solubility, large amounts of carbon dioxide can move
between air and liquid compartments even with small partial pressure
gradients.
We can be significantly affected by differences in the partial pressures of the
air we are breathing. At high altitudes, the fraction of oxygen in the
atmosphere is the same (21%), but the total atmospheric pressure is less (as
shown earlier). This causes the partial pressure of the oxygen we breathe in to
be significantly less than that at sea level, making it much harder to move
oxygen from the air into our plasma.
Example
Decompression Sickness
Scuba divers involved in deep dives breathe in air that is under very high
pressure. At sea level very little nitrogen enters our plasma since it has a
very low partial pressure. However, a large amount of nitrogen can
move into the plasma (and consequently the other body and cell compartments)
during underwater dives since the air in the tank is under higher pressure
as well as the diver who experiences the pressure of the column of water on
their body. When the diver ascends slowly, the scuba apparatus
automatically lowers the total pressure on the air the diver is breathing.
The partial pressure of the nitrogen in the tank becomes less than the
partial pressure of the nitrogen inside the diver’s body. Consequently the
nitrogen that had moved from the air in the tank into the blook and tissues will now move
from the blood and tissues back into the alveoli and be removed when the diver exhales.
But if the diver ascends too quickly, the gas, which has a very low
solubility, comes out of solution and moves into the gas phase while still
in the divers body. The gas phase becomes manifest as bubbles trapped
in the divers tissues. The trapped bubbles can cause severe joint pains,
dizziness, shortness of breath, extreme fatigue, paralysis, and possibly
death. A hyperbaric (high pressure) chamber allows the diver to breathe air
at an elevated partial pressure. The high partial pressure forces the nitrogen back into its dissolved
liquid phase state. The barometric pressure of the chamber is then
decreased very slowly to allow the nitrogen to be released slowly through
the lungs.
Hyperbaric chambers can also be used to increase the partial pressure of oxygen
and enhance its ability to enter our body through the respiratory system or
through open wounds. Clinically high partial pressures of oxygen administered
locally are used to treat people with regional infections such as bacterial
gangrene infections sometimes found in the limbs of diabetics with poor blood
circulation. Bacterial gangrene is an infection caused by anaerobic bacteria.
These bacteria cannot survive in an oxygen-rich environment. The high partial
pressures of oxygen found in the hyperbaric chamber can force more oxygen into
the tissues infected by the bacteria. If the tissues in which the bacteria are
living become too aerobic, the bacteria may die.
 By Intermedichbo (Hyperbaric Chamber) CC-BY-3.0
By Intermedichbo (Hyperbaric Chamber) CC-BY-3.0
External Respiration
Air containing oxygen enters the alveoli by the process of ventilation (described
in a later section). The partial pressure of oxygen in the alveoli is slightly
lower than the partial pressure of oxygen found in the atmosphere. Air taken
into the lungs (tidal volume minus the volume of the conduction zone) mixes with
air that is already in the lungs (functional residual volume). ). Because gas
exchange is constantly occurring (even between breaths, when we hold our breath,
etc.), the air making up the functional residual volume has had some oxygen
removed from it and some carbon dioxide added to it. When a new tidal volume of
air is inhaled, the portion of this air that enters the alveoli mixes with the
functional residual volume and effectively lowers the fraction of oxygen in this
alveolar air.
Inhaled air at sea level typically has a partial pressure of oxygen near 160
mmHg. In the alveoli, the partial pressure of oxygen would vary with our
ventilation pattern, but typically equilibrates at about 105 mmHg. It is the
difference between alveolar oxygen partial pressure and the plasma oxygen
partial pressure that drives external respiration across the alveolar membrane.
Blood coming through the pulmonary arterial circulation is lower in oxygen, with
a typical partial pressure of 40 mmHg. The differential concentration gradient
for oxygen to move from the alveolar air into the capillary blood starts at
about 65 mmHg (105 mmHg in the alveoli minus 40 mmHg in blood), providing enough
of a difference in partial pressures for oxygen to diffuse from the aveoli into
the capillary. Diffusion will occur along the length of the pulmonary capillary
until the partial pressures come into equilibrium near 105 mmHg.
Example
Altitude Sickness
Altitude sickness, also called acute mountain sickness, can strike people
climbing to elevations above 8,000 feet (although it typically occurs only
at altitudes much higher than this). At elevations high above sea level,
there is the same percentage of oxygen (21%), but much less atmospheric
pressure. This lowers the partial pressure of the oxygen being inhaled so
less oxygen enters the body. If the body doesn’t adapt well, a person can
experience altitude sickness ranging from mild to severe forms. Mild to
moderate altitude sickness can cause nausea, vomiting, tachycardia,
shortness of breath with exercise, or difficulty sleeping. Mild to moderate
cases usually resolve themselves when the person descends to a lower
altitude. However, severe cases are another matter. They can result in
cyanosis, pulmonary congestion confusion and stupor, a cough with or without
blood, a gray or very pale complexion, the inability to walk a straight
line, if able to walk at all, and shortness of breath when at rest. These
cases require immediate evacuation to lower altitudes. Without treatment,
severe altitude sickness may result in death due to pulmonary complications
or brain swelling. The good news is that altitude sickness can be prevented.
Individuals who climb to extremely high altitudes, like Mount Everest,
should do so slowly to allow their bodies to become acclimated to the
atmospheric differences.
The partial pressure of oxygen in the system circulation is slightly lower. As
blood enters the left atrium of the heart, a small amount of the pulmonary blood
mixes with the bronchial blood that has nourished the lung tissue, lowering the
partial pressure so that the actual concentration of oxygen in about is 100
mmHg, which is a typical value measured in a sample of arterial blood.
The functioning of the pressure gradient for carbon dioxide works in reverse of
that for oxygen, with the carbon dioxide partial pressure being higher in the
pulmonary arterial blood than in the alveoli. Even with a much smaller gradient
for carbon dioxide than for oxygen, nearly as much carbon dioxide diffuses from
the blood to the alveoli as oxygen diffuses from the alveoli to the blood
because of the much higher solubility of carbon dioxide in the plasma.
| External respiration: pulmonary |
PO2 |
PCO2 |
| Pulmonary arteries leading to capillaries |
40 |
45 |
| Alveoli |
105 |
40 |
| Pulmonary veins |
100 |
40 |
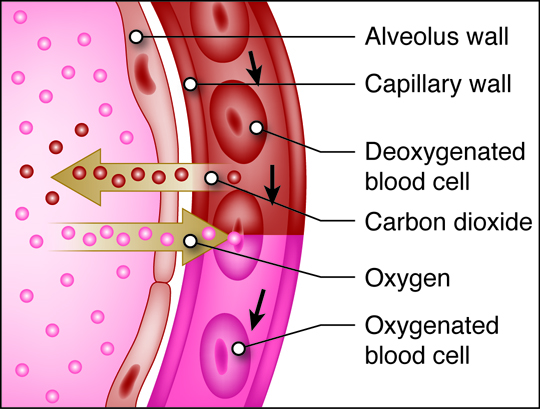 Deoxgynated blood cells. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Deoxgynated blood cells. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Internal Respiration
Internal respiration occurs between the blood and systemic tissues of the body.
The systemic arteries carry essentially the same concentration of oxygen and
carbon dioxide as the pulmonary veins. Oxygen is continually being used by the
tissues, and the partial pressure of oxygen in active cells remains below 40
mmHg. Oxygen circulating in the systemic arterial blood readily diffuses across
the membranes of the blood vessels into the tissues, replenishing the supply of
oxygen in the cells. The final concentration of oxygen and carbon dioxide in the
systemic veins is essentially the same as it is in the pulmonary arteries.
Just as the tissues are continually using up the oxygen, they are continually
producing carbon dioxide. The partial pressure of carbon dioxide in tissues is
always greater than 45 mmHg. This accounts for the diffusion of carbon dioxide
into the systemic capillaries, raising the pressure to 45 mmHg. The systemic
veins carry essentially the same concentration of oxygen and carbon dioxide as
the pulmonary arteries.
| Internal respiration: tissues |
PO2 |
PCO2 |
| Systemic arteries leading to capillaries |
100 |
40 |
| Metabolically active tissues |
< 40 |
> 45 |
| Systemic veins |
40 |
45 |
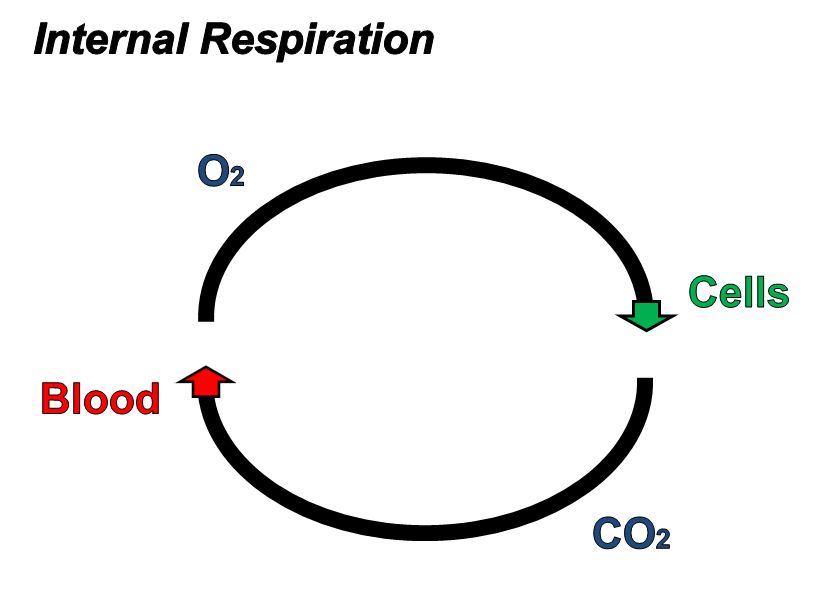 Oxgynated blood cells. This work by Cenveo is licensed under a Creative Commons
Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Oxgynated blood cells. This work by Cenveo is licensed under a Creative Commons
Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Mucus
Mucus is a slimy substance secreted by mucus membranes throughout the respiratory
system and other organ systems. Mucus formation in the nasal passages and upper
respiratory system, helps to moisten the air and to trap microorganisms and
particles. Throughout the entire respiratory system, mucus helps humidify and
buffer the cells that are in direct contact with air. Other organ systems
including in the digestive, urogenital, visual, and auditory systems use mucus
production to protect epithelial cells. While the molecular content varies
between organ systems, in general, mucus contains primarily water with enzymes,
immunoglobulins (and other immune proteins) salts, and high molecular weight
glycoproteins called mucins. The glycosylations on the proteins attract large
amounts of water. Consequently mucus serves as a means to maintain local levels
of hydration.
Surfactant
At the gas-liquid interface of the alveoli cell membranes, surfactants found in
the liquid surface layer lower surface tension. Surface tension arises when
water molecules hydrogen bond with each other. The hydrogen bonding of water
molecules makes it hard to “pull” the water molecules apart, which must to
happen for the alveoli to expand. If you have ever tried to pull to 2 wet glass
slides apart you have experienced surface tension created by the hydrogen
binding of water molecules. The molecules in the surfactant interrupt these
hydrogen bonds, making it much easier to expand the alveoli during inhalation.
The composition of surfactant is 80% phospholipids with the remaining fraction
made up of cholesterol and proteins. The alveoli themselves are so small that
without the surfactant, the surface tension created by the water on the internal
surface of the alveoli would force them to collapse during exhalation.
Example
Respiratory Distress Syndrome of the Newborn Infants.
Respiratory distress syndrome of newborn infants (RDS) most commonly affects
premature infants whose lungs have not developed fully, children born by caesarean
section, and children of diabetic mothers. The lungs of these infants cannot make
sufficient quantities of surfactant, making it very difficult for the affected
infants’ lungs to expand properly to breathe. Most cases of RDS occur in infants
born before 28 weeks gestation as the cells of the lungs responsible for surfactant
production, called type II alveolar cells, are generally not very active until later
in pregnancy.
Neonates with RDS struggle to breathe, leading to poorly oxygenated blood and
cyanosis (appearance of blue skin). Additional symptoms generally include apnea
(periods of breathing cessation) or rapid, shallow breathing. Laboratory procedures
can be done to determine the level of fetal lung maturity.
Treatment for RDS often involves administration of a higher fraction of inhaled
oxygen (above the normal 21%), or the use of a ventilator. Artificial surfactant can
be given, but is still considered experimental. If a premature birth is likely, the
expectant mother may be given a steroid such as cortisol to promote maturation of
the fetal lungs before birth. Prognosis is dependent upon the severity of the
disorder. Children often worsen within the first few days after birth, but then
usually recover with appropriate treatment.
Hemoglobin
The protein used to carry oxygen by nearly all vertebrates is hemoglobin. It
functions by binding oxygen in the capillaries of the lungs and carrying it
inside red blood cells. When the blood enters capillaries in tissues with a low partial
pressure of oxygen, the oxygen will leave the hemoglobin molecule and diffuse
into the tissue. One gram of hemoglobin can carry 1.34 ml of oxygen under normal
conditions. There are normally about 15 grams of hemoglobin in each deciliter of
blood meaning that each deciliter of blood has the potential to carry about 20
ml of oxygen. This is much greater than the 0.3 ml of oxygen that a deciliter of
blood can carry dissolved in plasma.
Hemoglobin is a complex compound containing two alpha and beta protein chain
pairs and four heme molecules that contain iron in its ferrous (Fe+3) state.
Each heme can bind one oxygen molecule, so a hemoglobin, because it contains 4
heme groups, can bind up to 4 oxygen molecules. When a hemoglobin molecule is
fully bound (saturated) with oxygen, we consider it to be an oxyhemoglobin. When
it is not fully saturated, it is typically referred to as deoxyhemoglobin, even
though it may still have oxygen bound to some of the heme groups. Note that even
though an individual hemoglobin molecule can only be 0%, 25%, 50%, 75% or 100%
saturated, we have nearly 300 million hemoglobin molecules in each red blood
cell. Collectively they can have any saturation level between 0% and 100%.
 Hemoglobin molecule. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Hemoglobin molecule. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
When oxygen binds to one heme group the affinity of the other binding sites
changes so that the other three heme groups more easily bind each additional
oxygen. Similarly, one heme group losing its oxygen makes it easier for the
other three to lose theirs. This characteristic of heme facilitates loading and
unloading of oxygen and helps to explain how oxygen can be so quickly attached
and detached from hemoglobin as it passes through the appropriate
capillaries.
Hemoglobin and Oxygen Association
Adult humans require about 250 ml of new oxygen to enter the bloodstream per
minute to support the internal respiration of their tissues while in a relaxed
state. Since oxygen is poorly soluble in our plasma, we only deliver about 15 ml
of oxygen per minute to our tissues in dissolved form (0.3 ml of dissolved
oxygen per deciliter of blood times 50 deciliters (a unit that refers to 10 mls
of a liquid) per minute of cardiac output. Therefore we become critically
dependent on another mechanism to help deliver sufficient amounts of oxygen to
where it is needed. That mechanism utilizes a protein, hemoglobin as a
transporter molecule for either oxygen or carbon dioxide. Red blood cells
contain high concentrations of hemoglobin.
In order to be able to discuss the different macromolecules and the reactions
they undergo, it is important to be familiar with the common chemical symbols
that are used.
Here are a few of symbols you will see:
- Deoxyhemoglobin (HHb) - A hemoglobin molecule that has been reduced and does
not have a full complement of oxygen molecules attached to it.
- Oxygen (O2) - A gas that is required for converting nutrients
into cellular energy. Oxyhemoglobin (HbO2)- A hemoglobin
molecules that has been oxidized and is bound to four oxygen molecules.
- Carbon dioxide (CO2) - A gas that is released as a waste product
during the breakdown of glucose to release energy.
- 2,3-bisphosphoglycerate (BPG) - is a molecule found in red blood cells that
can bind to hemoglobin and decrease its affinity for oxygen.
- Carbonic acid ( H2CO3 ) - is formed as an intermediate
step in the transportation of carbon dioxide. Carbonic anhydrase is an
enzyme that will speed up the formation of carbonic acid from water and
carbon dioxide.
- Bicarbonate (HCO3−) - Carbonic acid can quickly convert to
bicarbonate and a hydrogen ion. Bicarbonate plays a huge role in
transporting carbon dioxide and maintaining blood pH.
 Partial pressure. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Partial pressure. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
On this curve, the x axis lists the PO2 of the blood (whether it is in
the lungs or other body tissues), and the y axis lists the percent saturation of
the hemoglobin with oxygen. This graph has a sigmoidal ‘S’ shape, such that
changes in partial pressures above 80 mmHg do not have a major effect on the
percent of hemoglobin saturation. Since normal partial pressure in the lungs is
over 100 mmHg, there are some safety factors that are important for people who
travel to altitude or develop ventilation disorders to consider. As long as
neither is too severe, hemoglobin will stay close to 100% saturated as long as
the partial pressure in the alveoli can stay above 80 mmHg. Under normal
conditions, hemoglobin ends up nearly 100% saturated in the lungs and then
travels to the tissues. In the tissues, hemoglobin will have given up 25% of the
oxygen it carries such that it is now 75% saturated (the relationship between
oxygen levels and partial pressure is not linear because of the shape of the
curve). If there is a higher demand for oxygen in metabolically active cells,
more of the oxygen will diffuse into the tissues reducing hemoglobin saturation
below 75%.
Hemoglobin and Oxygen Dissociation
Tight binding of oxygen to hemoglobin allows us to transport oxygen throughout
our cardiovascular system. At the same time hemoglobin cannot bind oxygen so
tightly that oxygen cannot leave the hemoglobin when it the molecule encounters
oxygen deprived tissues. Metabolically active tissues cause local environmental
changes making it easier for oxygen to unload from hemoglobin in these tissues.
These changes in rates of dissociation can be visualized by looking at the
oxygen-hemoglobin dissociation under different conditions such
as changing temperature, PCO2, pH, and the levels of
2,3-bisphosphoglycerate (BPG). Increased cellular metabolism causes increases in
temperature, BPG production and carbon dioxide levels. The increased carbon
dioxide further causes a decrease pH. When this happens, oxygen more readily
leaves hemoglobin molecules, and the oxygen-hemoglobin dissociation curve is
shifted to the right. Regulation of oxygen delivery to cells is important since
cells with a high metabolic rate need more oxygen to produce ATP.
 Dissociation curve. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Dissociation curve. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Specifically, the oxygen-hemoglobin bond is weakened in the more acidic
environment, and the oxygen leaves the heme more readily. The most commonly
found acids that result in a locally lowered pH include lactic acid and carbonic
acid. Lactic acid production results from situations where there is inadequate
oxygen supply and cells have shifted to anaerobic metabolism of glucose for
energy production. Carbonic acid is formed when carbon dioxide dissolves in
water. Since carbon dioxide production is a byproduct of aerobic metabolism, it
follows that very metabolically active cells will exhibit increased levels of
carbon dioxide production. The chemical equation linking the increased carbon
dioxide to a lowering of pH is CO2 + H2O ↔
H2CO3 ↔ H++HCO3 which we will discuss
later.
Red bloods metabolize glucose in a variation of the standard glycolysis pathway.
BPG is an intermediate compound made in red blood cells during glycolysis. When
present in red blood cells, it attaches to the terminal amino acid groups of
hemoglobin’s beta chains and decreases the affinity of hemoglobin for oxygen.
BPG will increase in response to endocrine regulators including thyroxine,
epinephrine, norepinephrine, and testosterone, and as part of the compensation
that occurs at high altitudes and with some anemias.
Forms of Carbon Dioxide
Carbon dioxide is carried in the blood in three forms: dissolved, attached to
hemoglobin, and converted to bicarbonate ions. Dissolved CO2 accounts
for 7–10 percent of the carbon dioxide carried in the blood. This is also the
only form of carbon dioxide that diffuses from the tissues into the blood and
from the blood into the alveoli for expulsion from the body. Here, we will
examine in depth the transfer of carbon dioxide using hemoglobin or by the
formation of bicarbonate.
Carbaminohemoglobin and Bicarbonate
Carbon dioxide can bind to any protein and form a carbamate compound. The protein
found in the highest concentration in red blood cells is hemoglobin, and 20–23
percent of the CO2 carried in the blood is bound to hemoglobin in the
form of carbaminohemoglobin. In the capillaries of the systemic
tissues, CO2 molecules attach to the terminal amino acids of the
alpha and beta chains of the hemoglobin molecule. Deoxygenated hemoglobin
(hemoglobin with no or less than the maximal oxygen bound, abbreviated HHb),
such as that found in metabolically active tissues, binds CO2 easily.
In the capillaries of the lungs, the elevated levels of oxygen found in alveoli
force the carbon dioxide off the hemoglobin molecule and oxidize the protein,
freeing up hydrogen ions. Although some carbon dioxide is transported as
carbaminohemoglobin, the majority, about 70 percent, is dissolved in the blood
as bicarbonate ions that arise from the reversible reactions discussed below.
Carbon dioxide in the presence of water can be reversibly converted to carbonic
acid Carbonic acid is not very stable and readily dissociates into a hydrogen
ion and a bicarbonate ion. In fact, this is why carbonated beverages are acidic.
Carbon dioxide is added to the drink mixture under pressure and dissolves in the
beverage. When the CO2 has bubbled out of the beverage, it tastes
flat because the acid is gone. The same thing happens in red blood cells, except
that red blood cells contain an enzyme called carbonic anhydrase
(CA), which is capable of facilitating one million reactions per second per
enzyme molecule. Because of the enzyme, most of the CO2 dissolved in
the blood is quickly converted to carbonic acid which breaks down to form,
hydrogen ions, and bicarbonate ions.
The chemical reaction for this process is the following:
Where H2O is water, CA is carbonic anhydrase,
H2CO3 is carbonic acid, H+ is a hydrogen ion, and
HCO3- is a bicarbonate ion. The second part of the reaction,
which produces the hydrogen and bicarbonate ions, does not have an enzyme, but
depends on the dissociation of the weak acid. This series of reactions provides
buffering for the blood.
Carbon dioxide production occurs in many tissues, especially muscle. The carbon
dioxide amount of diffuses from the tissue of origin into a systemic capillary
and dissolves in the plasma. A small amount of the carbon dioxide is transported
this way. Most of the CO2 that diffused into the plasma diffuses into
a red blood cell and reacts with intracellular water molecules to produce
hydrogen and bicarbonate ions. Remembering that the reactions are reversible, it
makes sense that the direction of the reaction sequences will be driven by
levels of accumulated products. The dissociation of carbonic acid is driven by
the relative concentration of carbonic acid compared to the relative levels of
bicarbonate(carbonic acid’s conjugate base). A build-up of bicarbonate in the
RBCs would slow or halt the dissociation of carbonic acid. This build-up doesn’t
usually happen because, RBCs have a membrane channel that allows bicarbonate to
leave the RBC and enter the blood plasma. To maintain electric neutrality inside
the RBC and in the plasma, every time a negative bicarbonate ion leaves the red
blood cell it is exchanged for a negative chloride ion from the plasma. This
exchange is called the chloride shift. The bicarbonate ion in the
plasma becomes part of the blood’s buffering system, maintaining blood pH within
a narrow range. Deviation from this range compromises organ function and can
cause death. The hydrogen ion liberated from the conversion of CO2 to
bicarbonate binds to a a deoxygenated hemoglobin molecule causing it to become
reduced. Deoxygenated hemoglobin easily picks up a molecule of CO2,
creating carbaminohemoglobin. Hemoglobin is an important buffering agent for the
hydrogen ions produced from the conversion of carbon dioxide to bicarbonate
ions. If this buffering did not occur, the intracellular fluid of the red blood
cell would become progressively more acidic, resulting in deterioration of cell
functions. Some CO2 from the tissues can be found as as bicarbonate
ions and dissolved CO2 in the plasma. The remainder of the carbon
dioxide is attached to hemoglobin or it is still in the carbonic acid form and
will stay in the red blood cells.
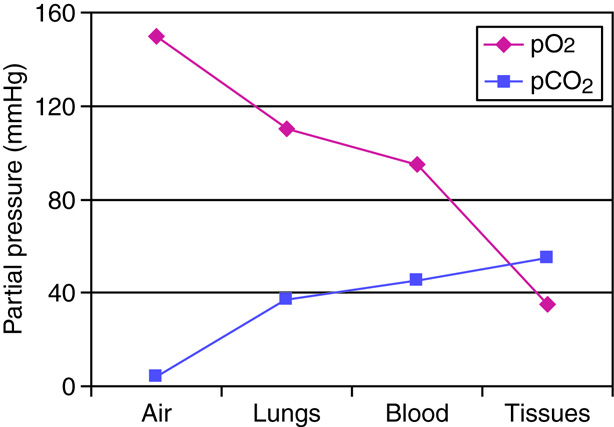 Graph of partial pressure. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Graph of partial pressure. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
When the blood enters the pulmonary capillaries, gaseous carbon dioxide in the
plasma diffuses into the alveoli. Some of the bicarbonate diffuses from the
plasma into the red blood cells, and a chloride ion passes back into the plasma,
reversing the chloride shift that occurred in the capillaries in the systemic tissues. The high partial
pressure of oxygen in the alveoli causes the carbaminohemoglobin to dissociate
into deoxyhemoglobin, a hydrogen ion, and a molecule of carbon dioxide. The
released CO2 is available for diffusion. The free hydrogen ion
combines with a bicarbonate ion and reforms carbonic acid. The carbonic acid is
converted back to carbon dioxide and water under the influence of carbonic
anhydrase.
Epithelial Cell Types
Respiratory epithelial cells include three types: a) ciliated cells, b) goblet
cells, and c) basal cells. Ciliated cells have cilia on the
surface, Goblet cells, shaped like a wine goblet, secrete mucus.
Basal cells, found at the basal region of epithelial sheets,
are stem cells used to regenerate and maintain a healthy epithelial cell
layer.
-
Cilia
The apical surfaces of ciliated epithelia cells possess specialized
structures called cilia. Depending upon the tissue cilia may function in
either sensory or mechanical capacities. The cilia in the nasal cavity
and bronchial tree sweep mucus toward the pharynx to be swallowed or
expectorated. Swallowing delivers the microorganisms and particles that
were trapped in the mucus to the stomach where the low pH of the stomach
will destroy them.
-
Macrophages in the lung
The respiratory system interacts with the external environment during gas
exchange. This interaction can provide contact with pathogens (viruses,
bacteria, and other disease causing organisms), atmospheric debris and
other particulates. We have already covered how the sticky mucus traps
many pathogens and particles and facilitates removal from the body. Yet
the mucus does not trap all inhaled particles. Particles that make it to
the level of the alveoli are typically removed by alveolar macrophages
through the process of phagocytosis.
-
Alveolar cells
In the alveoli there are specialized epithelial cells called alveolar
cells. Type I alveolar cells are very thin, simple squamous cells
through which gases easily diffuse. These cells are so thin that they
can only be seen through the use of an electron microscope. The type I
epithelial cells also make angiotensin-converting enzyme (ACE), an
important enzyme of the renin-angiotensin system used in the control of
blood pressure. Inhibition of this enzyme is one of the methods used to
control hypertension. Scattered among the squamous cells are type II
alveolar cells. Type II alveolar are cuboidal epithelial cells with
microvilli on their apical surface. These cells make and secrete
surfactant, which decreases the surface tension on the alveolar
surfaces. The alveoli themselves are so thin that without the surfactant
the force of surface tension created by the water on the cell surfaces
would cause the alveoli to collapse upon exhalation. Mixed in among the
types I and II alveolar cells are macrophages that migrate within the
sacs and clean up material that has entered this part of the system.
These cells may accumulate and sequester non-biodegradable material that
persists in the cells. Cells that contain accumulated material are
referred to as dust cells. The alveoli are considered to be functionally
sterile, due to the combination of the mucus and cilia found through
most of the respiratory membranes and the macrophages in the
alveoli.
Epithelial Types Throughout the Respiratory system
In the nasal cavities and upper respiratory tract, epithelial cells are
primarily ciliated psuedostratified columnar epithelium. The bronchial
tree is lined with pseudostratified, ciliated, columnar epithelia with
goblet cells dispersed among the columnar cells. At the terminal
bronchioles, epithelial become ciliated cuboidal cells without any
goblet cells.
Anatomy of the Respiratory-Cardiovascular Junction
There is intimate contact between respiratory tissue and the blood supply
in the lungs. Alveolar sacs in the lungs are wrapped in capillary beds
of the cardiovascular system. At the cellular level, the simple squamous
alveolar cells are in close contact with the capillaries, which are also
one endothelial cell thick.
Oxygen from the inhaled air diffuses from the alveoli to the hemoglobin
in the red blood cells. In order to do so, it has to diffuse through the
alveolar epithelial cell, the capillary endothelial cell, plasma in the
capillary, and into the red blood cell.
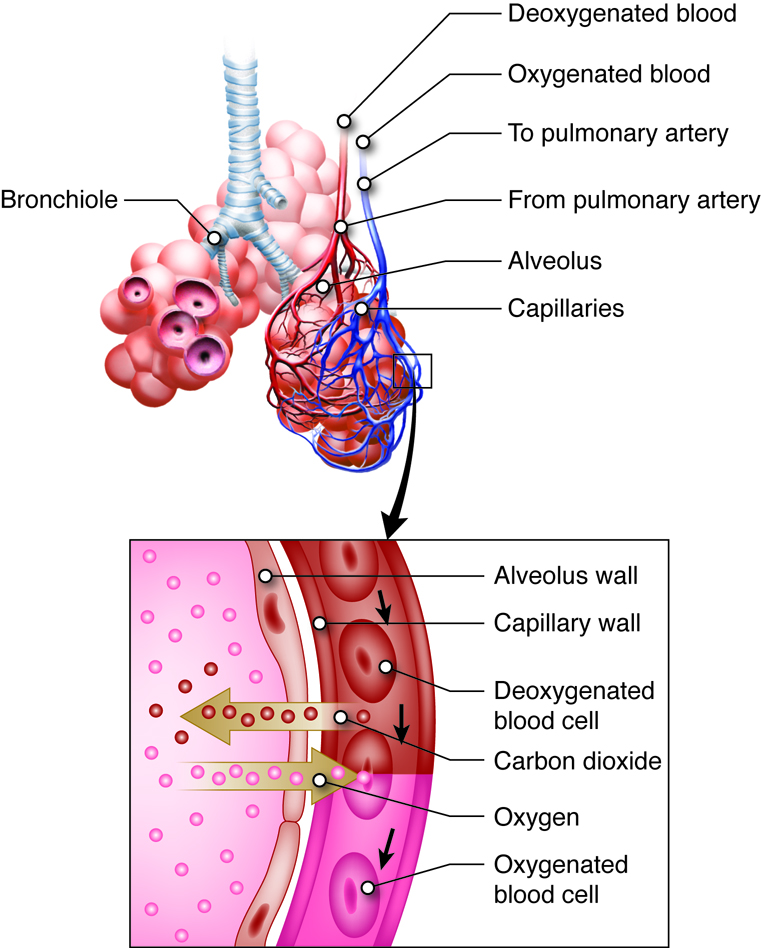 Bronchiole. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Bronchiole. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Let’s summarize
The major function of the respiratory system is to obtain oxygen needed to
convert nutrients to energy and to remove carbon dioxide that is a waste product
of this reaction. In order to perform this function the respiratory system must
have mechanisms for moving oxygen into the body from the atmosphere and a way to
transport the oxygen to tissues throughout the entire body. The respiratory
system must also transport carbon dioxide to the lungs to be expelled from the
body. Additionally, the respiratory system plays a role in protecting the body
from microorganisms and maintaining proper pH levels.
The primary function of the respiratory system, the exchange of oxygen and carbon
dioxide, occurs through several processes.
- Ventilation-moves air in and out of the lungs
- External respiration exchanges oxygen and carbon dioxide between the air and
the alveoli of the lungs.
- Internal respiration exchanges oxygen and carbon dioxide between the blood
and tissues.
- Cellular respiration uses oxygen to release energy from nutrients.
- To understand how the respiratory system functions, it is studied on the
chemical, macromolecular, cellular, tissue, organ and organ system
levels.
- The role of partial pressure in the diffusion of gases in external and
internal respiration
- Henry’s law discuss the solubility of gasses in a liquid and helps
explain why oxygen and carbon dioxide are transported
differently
- The macromolecule level related the structure of the macromolecule to
their functions
- Mucus- immunoglobulin for immune function, glycosylate proteins for
hydration
- Surfactant- phospholipids that disrupt the surface tension of water
in the alveoli to prevent collapse.
- Hemoglobin-iron containing heme group to transport oxygen and carbon
dioxide
- Transport of oxygen on hemoglobin the benefit of a tight
association and factors that will affect the affinity of
hemoglobin for oxygen
- Transport of carbon dioxide as carbaminohemoglobin and
bicarbonate as well as the role of carbonic anhydrase
- There are several important cell types that are found in different locations
of the respiratory system
- Epithelial cell types located in the respiratory tree include goblet
cells that secrete mucus, ciliated cells that sweep mucus into the
pharynx and basal cells which can regenerate and produce new layers
of cells.
- Macrophages located in the lungs will help to provide protection
against pathogens.
- Epithelial cells in the alveoli are divided into simple squamous Type I cells that
allow for diffusion and also secrete angiotensin-converting enzyme
and the cuboidal Type II cells that secrete surfactant.
In order to understand how the respiratory system functions, it is important to
understand how the different organizational levels function both independently
and together. Different portions of the respiratory system contain different
cell types that all work together to allow for the diffusion of oxygen and
carbon dioxide while providing protection from pathogens.
Homeostasis
The primary physiologic functions of the respiratory system are to provide oxygen
for cellular metabolic processes and to remove the gaseous waste product carbon
dioxide. When there is an increased need for oxygen, (best observed during
rigorous exercise), our respiratory system responds with an increased rate and
depth of breathing. In response to the adrenal hormone epinephrine, the
bronchioles will also dilate, making it easier to move this increased volume in
and out. With concurrent increases in cardiac output, we can typically meet our
increased demands of those tissues with increased metabolic rates. Most of us do
not make a conscious decision to increase our alveolar ventilation and the
sympathetic nervous system does not control the diaphragm. Dilating the airways
increases the rate at which air can move in and out, but does not affect
volume.
Even when there is no need for an increase in oxygen levels in the tissues, the
respiratory system will respond with increased ventilation if carbon dioxide
levels start to increase. Increased carbon dioxide levels can occur in various
forms of metabolic acidosis, where imbalances in metabolism lead to increased
carbon dioxide levels in the body. Increased ventilation helps rid the body of
carbon dioxide and limits the changes in pH the body would otherwise experience
with the rising carbon dioxide levels.
Example
Chronic Obstructive Pulmonary
Disease (COPD)
Chronic obstructive pulmonary disease (COPD) is a chronic, debilitating disease. COPD is
a set of symptoms that can develop as a result of either chronic bronchitis or
emphysema. People with chronic bronchitis constantly produce mucus in the conducting
division in response to inhaled irritants or mild infections Emphysema which is
permanent results from the progressive destruction of lung tissue. It is typically a
more severe form of COPD than bronchitis, and may lead to death. The leading cause of
both conditions is tobacco smoke, inhaled as either first-hand or second-hand smoke.
Occasionally, emphysema can develop as a result of exposure to gases or fumes in the
workplace. There is a low incidence of COPD resulting from a deficiency of the protein
alpha-1-antitrypsin.
The symptoms of COPD include a cough with or without mucus, fatigue, frequent respiratory
infections, shortness of breath (dyspnea), the inability to catch one’s breath, and
wheezing. As the disease progresses, patients may have more symptoms which can progress
in severity. Evaluating Lung sounds and X-rays are not necessarily useful in
establishing a diagnosis for COPD. Spirometry and the examination of arterial blood
gases to determine the blood concentrations of oxygen and carbon dioxide provide much
better diagnostic tools.
As COPD worsens, blood oxygen levels decrease and blood carbon dioxide levels increase.
The decreased oxygen leads to the fatigue, dizziness and decreased activity tolerance
these people often experience. The increase in carbon dioxide can lead to respiratory
acidosis, ultimately leading contributing to dysfunction in many of the body’s metabolic
pathways.
There is no cure for COPD, but medications can help alleviate its effects. Inhalers that
cause bronchodilation and contain steroids to reduce inflammation and mucus secretion
are effective in many cases. Other anti-inflammatory medications may also help. If the
conditions become severe, steroids can be administered orally or by intravenous methods.
Oxygen may be needed, and mechanical breathing assistance may be used.
Additional information is available at the following site:
http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001153
Integration of Systems
With the respiratory system’s constant interaction with our external environment
it is considered a portal of entry for irritants and microorganisms. Protection
of the body from these entering substances involves multiple forms of
defense.
The epithelia of the upper respiratory tract contains goblet cells that produce
mucus that lines the nasal cavity and most of the bronchial tree. The mucus
helps to moisten the air and to trap microorganisms and particles. Mucus also
contains immunoglobulin molecules which can bind pathogens andto signal immune
cells if needed. The cilia on respiratory epithelial cells of both the upper
(above the larynx) and lower (below the larynx) respiratory tracts beat toward
the pharynx creating the mucociliary elevator so that the mucus and trapped
material can be swallowed. Swallowing delivers the microorganisms and particles
to the stomach for destruction in stomach acid. Nerves and blood vessels
underlie the epithelia in the nasal cavity. Sensory receptors can be stimulated
by inhaled caustic material, resulting in the reflex called sneezing which rids
these materials from the respiratory tract. At the level of the alveoli,
phagocytic macrophages ‘patrol’ the lung tissues for invaders that have made it
deeper into the respiratory system.
Major Functions of the Urinary System
Usually, when we think of the urinary system, we think about getting rid of waste
products in our urine. The urinary system, however, involves more than just
waste removal. The urinary system plays many important roles in
the maintenance of homeostasis. This means this system helps to regulate the
internal conditions of the whole body. For instance, if the body is dehydrated, the body will
function to conserve the liquid. Consequently, the body does not produce large
volumes of urine. Much of this proper maintenance of homeostasis is a function
of the kidneys.
The roles of the urinary system include detoxifying harmful substances (or
toxins), maintaining water levels, maintaining appropriate levels of some
vitamins and minerals, maintaining acid-base and electrolyte balances, and
interacting with the circulatory system to help regulate blood pressure and red
blood cell count. In a three-way interaction with both the respiratory and the
circulatory systems, the urinary system helps stabilize blood oxygen and carbon
dioxide levels.
The final outcome of the above functions of the urinary system is excretion.
Excretion is the removal of wastes generated by the normal processes of cell
metabolism in the body. Such metabolic wastes include urea, uric acid,
creatinine, creatine, bilirubin, and ammonia. The metabolic wastes originate in
the cells throughout the body and are moved into the blood. If allowed to
accumulate, these wastes would be toxic to the body. All of the organs of the
urinary system are involved in the removal of these metabolic wastes by
contributing to the process of excretion. Other body systems that are also
involved in excretion are the respiratory system, integumentary system (the
skin), and the digestive system. Excretion and elimination are two similar
processes. Excretion specifically referes to the removal of the waste products
of metabolism from the body. Elimination is the explulsion of undigested or
unmetabolized waste products from the body.
Let's Summarize
The major functions of the urinary system include:
-
Excretion of waste products such as urea, uric
acid, creatinine, bilirubin, and ammonia.
-
Maintenance of homeostasis, or the ability for
the urinary system to regulate its internal conditions.
-
Detoxifying harmful substances in the
body
-
Maintenance of proper water levels, vitamin
and mineral levels, and acid-base and electrolyte levels
- Interaction with the respiratory and the circulatory systems, to help stabilize blood oxygen and carbon dioxide
levels.
Introduction to the Urinary Tract Anatomy
As noted above, much of the maintenance of proper chemical balance, or
homeostasis, is the function of the kidneys, which are located towards
the lower back. The process begins with waste carrying blood entering
each of the two kidneys through the renal artery. Urine is produced by
the nephrons in the kidneys. Once filtered, the blood exits through the
renal vein. Urine leaves each kidney through a ureter. Each ureter
transports the urine via peristalsis to the urinary bladder (a hollow,
muscular chamber that collects and stores urine). A single urethra
transports the urine from the urinary bladder to the outside of the
body. This process through the smooth muscles is otherwise known as:
peristalsis. An internal urethral sphincter muscle and an external
urethral sphincter muscle help keep the urine in the bladder until the
process of urination. The internal urethral sphincter muscle, surrounds
the neck of the urinary bladder at the juncture of the bladder with the
urethra. It opens reflexively when the bladder muscle contracts and
builds up pressure. The external urethral sphincter muscle is part of
the urogenital diaphragm and is located at the external opening of the
urethra. It is under voluntary control, and as a result of the voluntary
control, urination can be delayed for a time. Micturition is the entire
process of urination.
The process of urination is known as micturition which
begins with blood carrying various wastes that enter each of the two kidneys
through the renal artery and ends when urine exits out of the body through the
urethra.
To produce urine, nephrons and collecting ducts carry out three basic functions:
glomerular filtration, tubular reabsorption, and tubular secretion.
The first step in urine production is glomerular filtration. In this
process, water and most of the solutes in blood plasma pass through the wall of
glomerular capillaries, first into the glomerular capsule and then into the renal
tubule. The product of glomerular filtration is referred to as glomerular filtrate.
During tubular reabsorption, cells of the tubule reabsorb almost all water
and a variety of solutes from the filtrate as it flows through the renal tubule and
collecting duct. These reabsorbed substances are then returned to the blood in the
peritubular capillaries and vasa recta. In contrast to absorption, in which new
substances enter the body (e.g., through the gastrointestinal tract), reabsorption
refers to substances that have been removed from the blood being returned to the
blood.
In the process of tubular secretion, renal tubule and collecting duct actively transport
substances (e.g., wastes, drugs, excess ions) from the blood in the peritubular
capillaries. Secretion, in this case, refers to the removal of substances from the
blood. In contrast, the secretion of hormones or enzymes, for example, refers to cells
releasing substances into blood, ducts, and interstitial fluid.
The normal amount of filtrate produced as a result of glomerular filtration is so high
that the amount of fluid entering the proximal convoluted tubules in 30 minutes is
greater than the total blood plasma volume. Clearly, some of this fluid must be
reclaimed and sent back into the bloodstream to maintain water balance and ion
concentrations. This reclamation process occurs through reabsorption in the tubules.
Secretion is essentially the opposite of reabsorption. It removes the additional solutes
from the plasma and actively transports them to the tubule. Many students get confused
initially with this concept that secretion means end up in the urine and reabsorption
means ends up back in the blood.
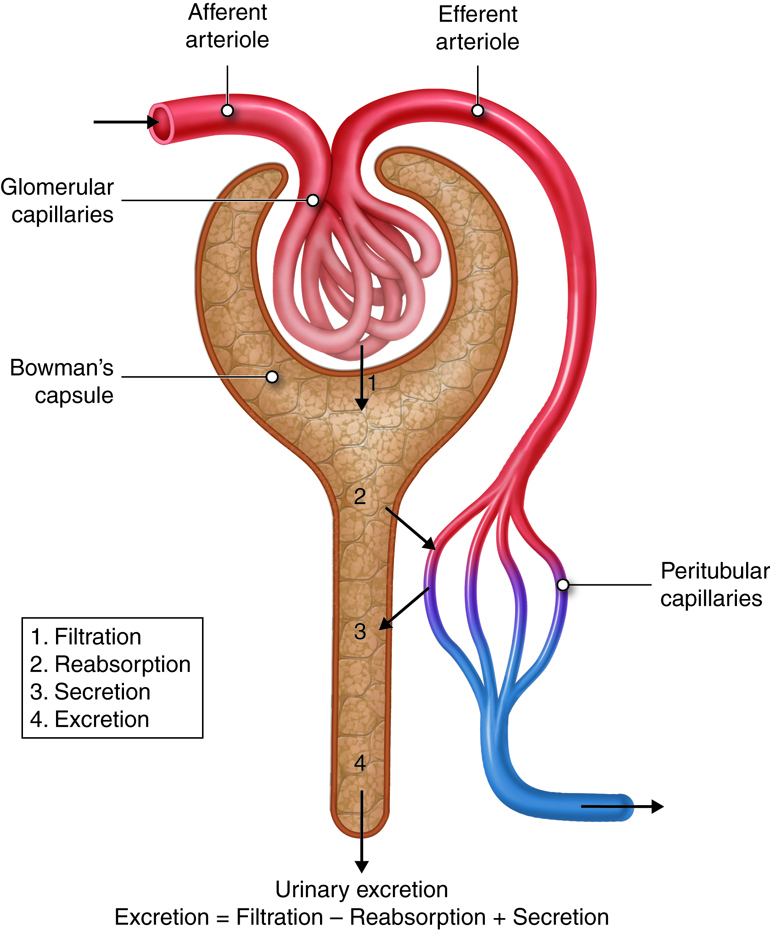 Reabsorption and Secretion. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Reabsorption and Secretion. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Glomerular Filtration
High Pressure Bulk Filtration
The glomerular capillaries filter about one fifth of the plasma that
flows through the kidneys into the renal tubules. Glomerular filtration
works like any other filtration process. For example, a coffee filter
prevents large coffee grounds from passing through it, while allowing
the passage of water and small solutes such as flavor molecules and
caffeine. In the same way, the glomerular filtration membrane blocks the
passage of blood cells and proteins, while allowing water and other
solutes from the blood to pass into the glomerular capsule. These
substances are forced through the membrane by the higher hydrostatic
pressure in the glomerular capillary than in the capsular space.
The glomerulus has the highest rate of filtration of any capillary bed in
the body for a number of reasons. First, its filtration membrane has a
very large surface area. In addition, the large
fenestrations (capillary pores) in glomerular
capillaries make them at least 50 times more permeable to water and
solutes than other capillary beds. Finally, the blood pressure
(hydrostatic pressure) in glomerular capillaries is about three times
higher than in other capillary beds (around 55 mm Hg versus 18 mm Hg or
less). This increased blood pressure is due to the relatively small
pressure drop across the afferent arteriole leading into
the glomerular capillary and the large pressure drop across the
efferent arteriole, which leads out of the glomerular
capillary. Looking at filtrate production, the combined daily output of
all other capillary beds in the body is about three to four liters
(three to four quarts). In the kidneys, the daily output is 150 liters
(158 quarts) in women and 180 liters (190 quarts) in men. Tubular
reabsorption returns more than 99 percent of the glomerular filtrate
(the fluid than enters the capsular space) to the bloodstream. This
means than just one to two liters are eliminated in urine. The
filtration fraction describes the portion of blood
plasma in the afferent arterioles that ends up as glomerular filtrate. A
typical filtration fraction ranges from 16 to 20 percent.
The Filtration Membrane
The porous filtration membrane separates the inside of the
glomerular capsule from the blood. Water and solutes smaller than about
three nanometers in diameter (e.g., glucose, amino acids, nitrogenous
wastes) pass freely across the membrane between the blood and capsule.
This means there are similar concentrations of these substances in both
the blood and the glomerular filtrate. Some bigger molecules may find a
way through the membrane, but molecules larger than five nanometers
(large proteins and blood cells) do not usually get into the tubule.
The filtration membrane, three layers serve as barriers to larger
molecules circulating in the blood. From most permissive to most
restrictive, these layers are the glomerular capillary endothelium, the
gel-like basal lamina basement membrane, and the podocyte-formed
filtration slit. All plasma components (note plasma does not include
blood cells) can pass through the fenestrations in the endothelium, but
blood cells cannot. The smallest proteins and most other solutes can
penetrate the basement membrane. This membrane is composed primarily of
negatively charged glycoproteins that oppose other macromolecular anions
and obstruct their entry into the tubule. In other words, the basement
provides electrical selectivity to the filtration process. Most plasma
proteins are too large to meet the size restrictions. Moreover, because
most plasma proteins have a net negative charge, they are repelled by
the negatively charged glycoproteins of the basement membrane. Any
macromolecules that do manage to pass through the basement membrane face
yet another barrier, thin membranes called slit diaphragms that span the
filtration slits. Specialized cells in the glomerulus, known as
mesangial cells, will destroy macromolecules that are trapped in the
filtration membrane. Mesangial cells can also contract and alter the
capillary surface area available for filtration.
Net Filtration Pressure
Three forces affect glomerular filtration rate (volume filtered per unit
time); hydrostatic pressure of the blood in the glomerulus, hydrostatic
pressure of the fluid in the capsular space, and colloid osmotic force
of the blood in the glomerulus. Hydrostatic pressure within the
glomerular capillaries promotes or enhances filtration. The other two
forces oppose filtration and are: back pressure from hydrostatic
pressure of the fluid within the capsule and colloid osmotic pressure
caused by the plasma proteins within the glomerular capillaries.
Subtracting the opposing pressures from the promoting pressure yields
the net filtration pressure.
Hydrostatic Pressure – Resistance from fluid in the tubule – Osmotic
Collodial Pressure = Net Filtration Pressure
Net Filtration Pressure
| Pressure |
Description |
Average |
Effect |
| Glomerular hydrostatic |
Glomerular capillary blood pressure |
55 mm Hg |
Promotes filtration |
| Capsular hydrostatic |
Hydrostatic pressure applied to the filtration membrane by fluid
in the capsular space and renal tubule |
15 mmHg |
Opposes filtration |
| Blood colloid osmotic |
Results from proteins present in blood plasma |
30 mmHg |
Opposes filtration |
The glomerular hydrostatic pressure (i.e., the blood
pressure in glomerular capillaries) is the primary force responsible for
pushing water and solutes from blood across the filtration membrane.
This unusually high pressure (55 mm Hg) is opposed by two forces that
resist the influx of fluids: the capsular hydrostatic pressure
exerted by fluid already in the glomerular capsule and the
blood colloid osmotic pressure caused by proteins
present in blood plasma (e.g., albumen, fibrinogen). Net filtration
pressure averages 10 mm Hg (i.e., 55 mmHg −15 mmHg − 30 mmHg = 10 mmHg).
This is in contrast to the 0.3 mmHg net pressure found in most
capillaries of the body.
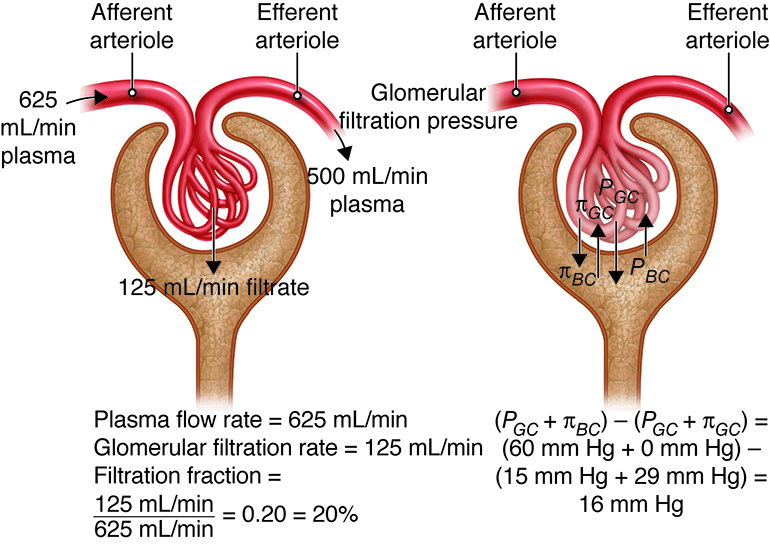 Glomerular filtration process. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Glomerular filtration process. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The glomerular filtration rate (GFR) is the total amount of
filtrate formed by the two million renal corpuscles in the kidneys
divided by time. The average GFR is 125 milliliters (4 ounces) per
minute, which adds up to nearly 140 liters (50 gallons) a day. The
kidneys must maintain a relatively consistent GFR to prevent homeostatic
imbalances in body fluids. If the GFR is too high, needed substances
will rush through the renal tubules too quickly to be completely
absorbed, and they will be lost in urine. A GFR that is too low will
allow waste products to accumulate in the plasma, ultimately leading to
illness and death if not corrected.
Tubular Reabsorption
Reabsorption starts as soon as filtrate enters the proximal convoluted tubules.
Before the reabsorbed substances can reenter the blood, they must pass through
three barriers: the luminal and basolateral membranes of cells in the tubule,
and the endothelium of the peritubular capillaries.
Reabsorption returns about 99 percent of filtered water and many filtered solutes
to the bloodstream. Although cells in the proximal convoluted tubule perform
most reabsorption, epithelial cells all along the length of the renal tubule and
the collecting duct also contribute to this process. To maintain normal plasma
levels, almost every organic nutrient (e.g., glucose, amino acids) is completely
reabsorbed. The amount of water and number of ions reabsorbed is determined by
hormonal signals and is based on homeostatic needs. The scope of reabsorption
can be appreciated by looking at water. Each day, about 180 liters (190 quarts)
of water are filtered in the kidneys. Only 1–2 liters (1–2 quarts) are excreted
in urine; the rest is reabsorbed in the kidneys.
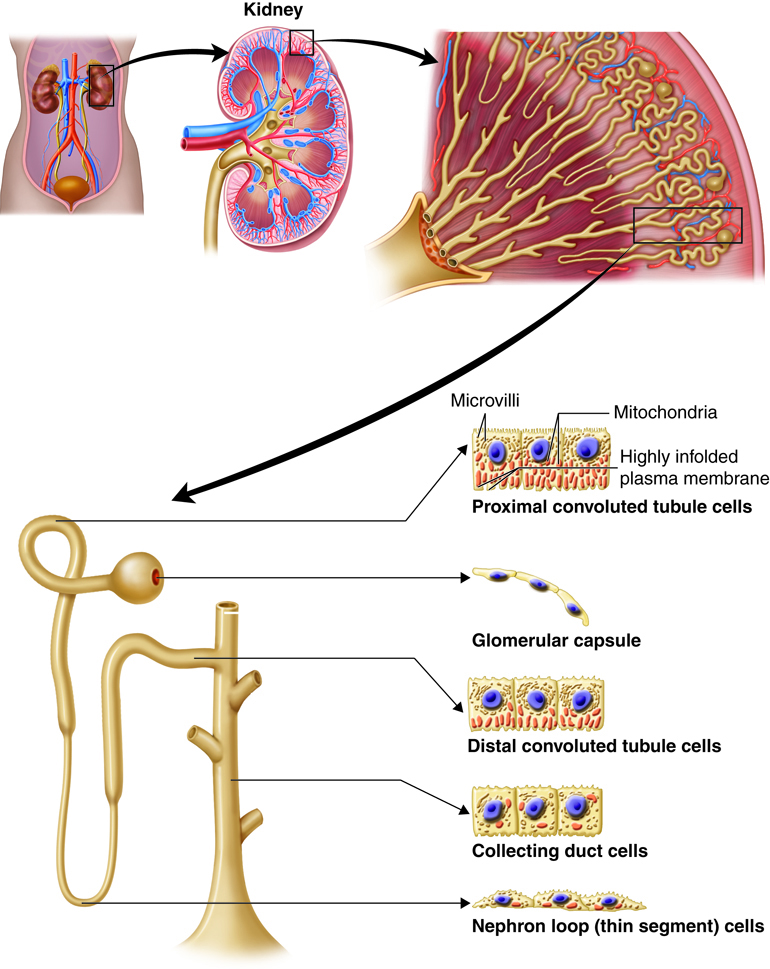 Tubules. This work by Cenveo is licensed under a Creative Commons
Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Tubules. This work by Cenveo is licensed under a Creative Commons
Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
In the table below, review the partial list of substances filtered through the
kidneys. As you can see, for most of these substances very little is excreted in
the urine. For example, only .03 grams of the 275 filtered grams of Bicarbonate
ions are excreted daily while none of the glucose that is filtered through the
kidneys is excreted. On the other hand, a larger proportion of the filtered Urea
is excreted when compared to the other substances.
Reabsorption and Excretion of Substances in the Glomerular
Filtrate
| Substance |
Filtered* (grams/day) |
Reabsorbed (grams/day) |
Excreted in Urine (grams/day) |
| Bicarbonate ions |
275 |
274.97 |
0.03 |
| Chloride ions |
640 |
633.7 |
6.3 |
| Creatinine |
1.6 |
0 |
1.6 |
| Glucose |
162 |
162 |
0 |
| Potassium ions |
29.6 |
29.6 |
2.0** |
| Proteins |
2.0 |
1.9 |
0.1 |
| Sodium ions |
579 |
575 |
4 |
| Urea |
54 |
24 |
30*** |
| Uric acid |
8.5 |
7.7 |
0.8 |
It is not necessary to memorize the numbers, but note the
importance of reabsorption.
*Refers to the amount entering the glomerular capsule, assuming
GFR is 180 liters (190 quarts) per day.
**After almost all filtered potassium ions are reabsorbed in the
convoluted tubules and nephron loop, principal cells in the collecting
duct secrete some of these ions.
***Urea is also secreted (in addition to being reabsorbed and
excreted).
Reabsorbed substances can be returned to the bloodstream to maintain homeostasis
by going between tubule cells (paracellular reabsorption) or by
going through individual cells (transcellular reabsorption) on
their way to entering a peritubular capillary. Cells in the renal tubule are
joined together at tight junctions. Although these junctions are tight, they are
just leaky enough to let some solutes pass through during paracellular
reabsorption. Substances reabsorbed through the transcellular route move through
the tubule cell's apical membrane, then through the intracellular fluid, and
finally through the basolateral membrane to reach the interstitial fluid. The
apical membrane is in contact with the tubular fluid, and the
basolateral membrane is in contact with interstitial fluid as
well as the bottom and sides of cells.
The apical and basolateral membranes of renal cells contain a variety of
transport proteins. The mechanism of transport depends on the substance being
reabsorbed. In passive reabsorption, no ATP is required. In active reabsorption,
the energy derived from the hydrolysis of ATP helps "pump" the substance across
a membrane—either directly or indirectly—during at least one step in the
reabsorption process.
Because all water is reabsorbed via osmosis, the reabsorption of water is coupled
with the reabsorption of solutes. About 90 percent of water reabsorbed in the
kidneys is accompanied by the reabsorption of sodium ions, chloride ions,
glucose, and other solutes. Because the water is "obliged" to tag along with the
reabsorbed solutes, this process is called obligatory water
reabsorption. It occurs in the water-permeable proximal convoluted
tubule and descending limb of the nephron loop. The remaining 10 percent of
water is reabsorbed through facultative water reabsorption, which
is controlled by antidiuretic hormone and takes place primarily in the
collecting ducts.
When filtrate enters the proximal convoluted tubule, it is referred to as
tubular fluid or tubular filtrate. The composition
of the tubular fluid changes as it travels through the tubule and collecting
duct, as each segment reabsorbs different substances.
Reabsorption in the Proximal Convoluted Tubule
The majority of solute and water reabsorption occurs in the proximal
convoluted tubule. Sodium ions, which are the most plentiful cation
(positively charged ion) in the tubular fluid, participate in most
solute reabsorption. Sodium ion carriers co-transport all filtered
glucose, amino acids, lactic acid, water-soluble vitamins, and other
nutrients. The reabsorption of sodium ions into the interstitial fluid
and then into the blood creates the osmotic gradient needed for water
reabsorption. An osmotic gradient is produced when the
solute concentration in the interstitial fluid is higher than that in
the tubular fluid.
| Region |
Reabsorbed Substances |
Amount Absorbed (Percent)
|
Mechanism |
| Proximal convoluted tubule |
Water |
65 |
Obligatory water reabsorption |
|
Sodium ions (Na+) |
65 |
Primary active transport |
|
Potassium ions (K+) |
65 |
Passive transport; paracellular route |
|
Glucose, amino acids, and most other organic solutes |
100 |
Secondary active transport with Na+ |
|
Chloride ions (Cl−) |
50 |
Passive transport; paracellular diffusion |
|
Bicarbonate ions (HCO3−) |
80-90 |
Secondary active transport with Na+ |
|
Urea |
50 |
Passive diffusion |
|
Calcium ions (Ca2+) |
variable* |
Passive transport; paracellular route |
|
Magnesium ions (Mg2+) |
variable* |
Passive transport; paracellular route |
*Amount reabsorbed depends on the needs of the body.
Reabsorption in the Nephron Loop
Because all glucose, amino acids, and other nutrients are reabsorbed in
the proximal convoluted tubule, fluid flows into the nephron loop (loop
of Henle) at a rate of 40 to 45 milliliters per minute (mL/min) (1.5
ounces/min), down from 125 mL/min (4.5 ounces/min) in the proximal
convoluted tubule. The chemical composition of the tubular filtrate at
this point is very different from that of the glomerular filtrate even
though the osmolarity is similar. The fluid's osmolarity is still
comparable to that of blood, because water was reabsorbed as solutes
were reabsorbed throughout the proximal convoluted tubule. Since part of
the nephron loop is comparatively water-impermeable, the reabsorption of
water in this region is not necessarily linked to the reabsorption of
solutes. Water is reabsorbed by osmosis in the water-permeable
descending limb, but not in the ascending limb. This water reabsorption
in the water-permeable descending limb can occur because the descending
limb dips down toward, or into, the renal medulla, where the osmolarity
of the interstitial fluid increases. This creates a gradient for this
water reabsorption to occur. Essentially no solutes are reabsorbed in
the descending limb, while solutes are reabsorbed via both active and
passive mechanisms in the ascending limb. The permeability differences
in these two limbs plays an important role in the ability of the kidneys
to form either dilute or concentrated urine.
| Region |
Reabsorbed Substances |
Amount Absorbed(Percent)
|
Mechanism |
| Descending limb |
Water |
15 |
Obligatory water reabsorption |
| Region |
Reabsorbed Substances |
Amount Absorbed(Percent)
|
Mechanism |
| Ascending limb |
Na+ |
20–30 |
Secondary active transport; paracellular diffusion |
|
K+ |
20–30 |
Secondary active transport; paracellular diffusion |
|
Cl− |
35 |
Secondary active transport; paracellular diffusion |
|
HCO3− |
10–20 |
Secondary active transport with Na+ |
|
Ca2+ |
variable* |
Passive paracellular diffusion |
|
Mg2+ |
variable* |
Passive paracellular diffusion |
*Amount reabsorbed depends on the needs of the body.
Reabsorption in the Distal Tubule and Collecting Duct
Because 80 percent of filtered water is reabsorbed by the time tubular
filtrate enters the distal convoluted tubules, the flow of this fluid
has slowed to a rate of approximately 25 mL/min (1 ounce/min). Most
reabsorption in this segment occurs in the initial portion of the distal
convoluted tubule. When the fluid reaches the end of the distal tubule,
90 to 95 percent of solutes and water have been reabsorbed. Both
principal and intercalated cells located here and in the collecting
duct. Principal cells reabsorb sodium ions (and secrete potassium ions),
while the intercalated cells reabsorb potassium ions and bicarbonate
ions (and secrete hydrogen ions). The needs of the body determine how
much of these solutes, and of water, are reabsorbed in the late distal
convoluted tubule and collecting duct.
| Region |
Reabsorbed Substances |
Amount Absorbed(Percent)
|
Mechanism |
| Distal convoluted tubule |
Water |
10–15 |
Obligatory water reabsorption |
|
Na+ |
5 |
active Na+ transport |
|
Cl− |
5 |
active Na+ transport |
|
Collecting duct |
|
|
|
Water |
variable* |
Facultative water reabsorption; antidiuretic hormone required to
insert aquaporins (water channels) |
|
Na+ |
3 |
Primary active transport (requires aldosterone) |
|
Na+, H+, HCO3−, Cl−, hydrogen ions (H+) |
variable* |
Primary active transport of Na+ and the medullary gradient
create the conditions for passive transport of some HCO3− and
Cl− and co-transport of H+, Cl−, and HCO3− |
|
K+ |
variable* |
K+ is both reabsorbed and secreted (aldosterone dependent),
usually resulting in net K+ secretion |
|
Urea |
variable* |
Facilitated diffusion in response to concentration gradient in
the deep medulla region; recycles and contributes to medullary
osmotic gradient |
*Amount reabsorbed depends on the needs of the body.
Tubular Secretion
There are two methods of removing unwanted substances from filtrate. First,
tubular cells simply do not reabsorb some solutes. The second method is
tubular secretion. Secretion transfers unwanted substances from
the blood and tubule cells into the tubular fluid. With the exception of
potassium ions, most secretion occurs in the proximal convoluted tubule.
However, some secretion also occurs in the cortical regions of the collecting
ducts and in the late distal convoluted tubule.
Tubular secretion is important for a number of reasons. It removes substances
that are not easily filtered, such as some drugs and metabolites that are
securely attached to plasma proteins. Second, it disposes of unwanted substances
or end products that have been reabsorbed passively, such as urea and uric acid.
Third, it eliminates excess potassium ions. Finally, it regulates blood pH. As
an example, when blood pH becomes too acidic, secretion adds more hydrogen ions (acid)
into the filtrate and retains more bicarbonate ions (a base).
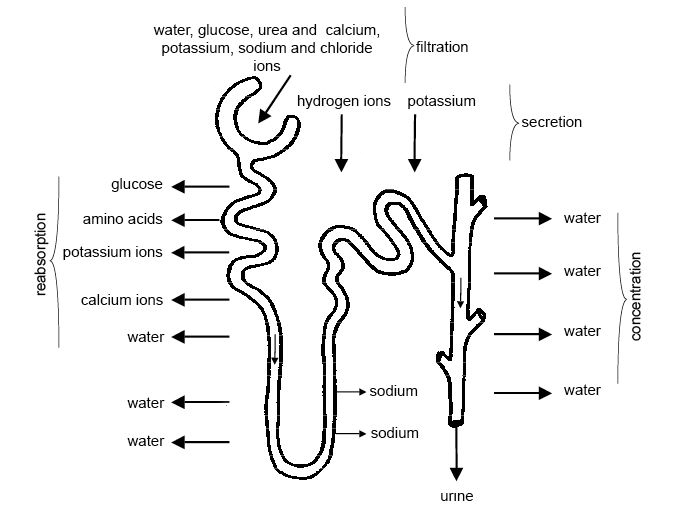 By Sunshineconnelly at en.wikibooks from Wikimedia Commons is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
By Sunshineconnelly at en.wikibooks from Wikimedia Commons is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Secretion in the Proximal Convoluted Tubule
With the exception of potassium ions, the proximal convoluted tubule is
the primary site of secretion. This tubule secretes a range of organic
ions, including hydrogen ions and ammonium ions. Ammonium ions are a
poisonous waste product of deamination (the process that removes an
amino group from an amino acid). Urea is also secreted in this
tubule.
Secretion in the Distal Convoluted Tubule and Collecting Duct
Secretion and reabsorption of sodium, potassium, hydrogen, and
bicarbonate ions are regulated and adjusted depending on various
circumstances. For instance, the rate of potassium ion secretion is
adjusted for the dietary intake of potassium, with the goal of
maintaining a stable potassium ion level in body fluids. Potassium ions
are regularly being brought into principal cells by basolateral
sodium-potassium pumps. This process results in a high
intracellular concentration of potassium ions. There are leakage
channels in the apical (luminal) membrane of principal cells through
which sodium ions enter and potassium ions escape. This mechanism of
secretion is the primary source of the potassium ions that are excreted
in the urine.
Reabsorption and Secretion Summary
Reabsorption removes useful substances from the glomerular filtrate and returns
them to the blood. The proximal convoluted tubule is the primary site of
absorption; it is where all glucose, most amino acids, and the majority of salts
are reabsorbed. Water reabsorption is coupled with the reabsorption of solutes
in the proximal convoluted tubule in a process called obligatory water
reabsorption. Only about 10 percent of water is reabsorbed in the collecting
ducts, with the help of antidiuretic hormone, in a process called facultative
water reabsorption. Secretion removes unwanted substances from the filtrate,
such as urea and uric acid, so they can be excreted in urine. Secretion also
helps regulate blood pH by adjusting the hydrogen ion and bicarbonate ion
content of the filtrate. Several hormones affect reabsorption and secretion.
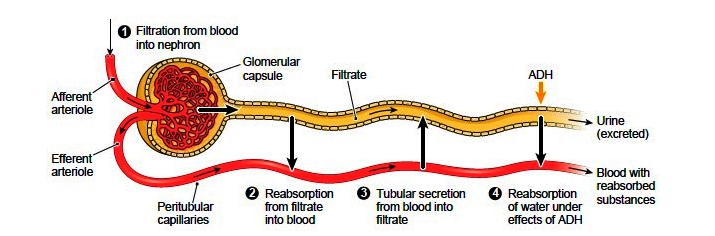 Filtration Summary.
Filtration Summary.
The process of osmosis plays a major role in the regulation of urine concentration.
Recall that osmosis refers to the flow of a liquid across a semipermeable membrane to
equalize the solute concentration on both sides of the membrane. The ability of the
solution to cause osmosis, its osmotic activity, depends on the amount and not the type
of solute particles that cannot pass through the semipermeable membrane. For example, in
the same volume of water, the osmotic activity of 10 sodium ions is the same as that of
10 glucose molecules, and the same as 10 proteins, and so on. Thus, the osmotic activity
of a solution is based on the osmolarity of that solution, a quality
defined as the number of solute particles dissolved in one kilogram of water. Osmotic
activity is frequently measured in units of 0.001 mole of particles, a millismol (mOsm).
Osmolarity is measured in mOsm/liter.
Formation of Dilute Urine
The ratio of water and solutes in glomerular filtrate is the same as that in
blood, with an osmolarity of around 300 mOsm/liter (one mOsm is translated as a
milli osmole or one thousandth of an osmole). The osmolarity of the filtrate
that enters the proximal convoluted tubule is still the same. And when filtrate
enters the nephron loop's descending limb, its osmolarity is the same as both
blood plasma and cortical interstitial fluid. Because tubular fluid is already
dilute as it flows through the ascending limb of Henle's loop, the formation of
dilute urine is a relatively simple process. When the hypothalamus (via the
posterior pituitary gland) is not secreting ADH, additional water is not
reabsorbed in the collecting ducts and dilute urine is produced. Before urine
enters the renal pelvis, it is further diluted by the removal of ions, including
sodium ions, in the distal convoluted tubule and collecting duct. As a result,
the osmolarity of urine can be reduced to nearly one fourth of that in the
glomerular filtrate or plasma, dropping to as low as 70 mOsm.
Formation of Concentrated Urine
If a person does not drink enough water, sweats a lot, or has diarrhea, water
conservation is required. In such cases, the kidneys will produce a small amount
of concentrated urine to preserve water, while still eliminating wastes and
surplus ions. Antidiuretic hormone (ADH), as its name suggests, inhibits
diuresis (urine production). ADH helps the kidneys generate urine that is up to
four times as concentrated as glomerular filtrate or blood plasma (up to 1,200
mOsm/liter for urine versus 300 mOsm/liter for glomerular filtrate). ADH can do
this only when there is an osmotic gradient of solutes in the renal medulla's
interstitial fluid, in other words, when the solute concentration in the
interstitial fluid is higher than that in the tubular fluid. This osmotic
gradient, coupled with the insertion of aquaporins into the luminal membrane of
the collecting ducts' principal cells, causes water and urea to pass from the
filtrate into the interstitial space to equalize osmolarity. At maximum ADH
secretion, as much as 99 percent of the water in the tubular filtrate is
reabsorbed, and the kidneys produce about half a liter of highly concentrated
urine per day. The production of concentrated urine is key to our ability to
survive for an extended period of time without water.
The osmotic gradient needed to produce concentrated urine depends on two chief
factors. First, individual areas of the nephron loop differ in permeability and
reabsorption characteristics. Second, fluid flows in opposite directions through
adjacent tubes in different parts of the urinary system. This process is called
countercurrent flow of fluid. It occurs down and up the
descending and ascending limbs of the nephron loop. Blood flowing along the
ascending and descending portions of the vasa recta also follows countercurrent
flow. Two countercurrent mechanisms operate in the kidneys: countercurrent
multiplication and countercurrent exchange.
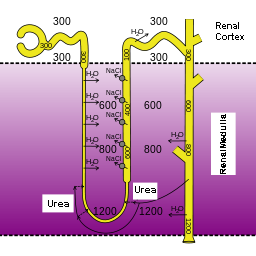 By Yosi I(Hypertonic Urine 1) CC-BY-3.0
By Yosi I(Hypertonic Urine 1) CC-BY-3.0
Countercurrent Exchange
In countercurrent exchange, countercurrent flow enables the passive
exchange of water and solutes between blood in the vasa recta and the medullary
interstitial fluid. The osmolarity of blood entering the vasa recta is around
300 mOsm/liter. In its descending limb, urea and sodium and chloride ions
diffuse from the increasingly concentrated medullary interstitial fluid into the
blood, while water diffuses from the blood into the interstitial fluid. Along
the ascending limb of the vasa recta, the concentration of the interstitial
fluid steadily decreases. At this point, urea and sodium and chloride ions
diffuse from the blood back into the interstitial fluid, and water diffuses in
the opposite direction. Because the osmolarity of blood leaving the vasa recta
is only a little higher than that of blood leaving it, it can provide oxygen and
nutrients to the renal medulla without washing out the osmotic gradient. To
summarize, the osmotic gradient in the renal medulla is created by
countercurrent multiplication in the nephron loop, and it is maintained by
countercurrent exchange in the vasa recta.
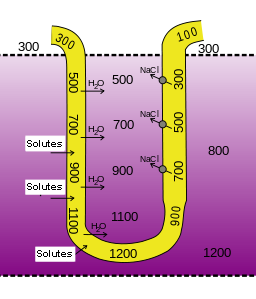 By Yosi I(Hypertonic Urine 2) CC-BY-3.0
By Yosi I(Hypertonic Urine 2) CC-BY-3.0
Countercurrent Multiplication
The nephron loop is responsible for the countercurrent multiplication
mechanism that establishes an osmotic gradient within the medullary
interstitium. This gradient is necessary for the collecting duct to create an
osmotic gradient.
Three factors are involved in countercurrent multiplication. First, recall that
the ascending limb is responsible for the active transport of sodium, chloride
and potassium out of the tubular fluid and into the interstitium. Because this
limb is relatively water impermeable, the solutes move into the interstitial
fluid without additional water, increasing the osmotic pressure of the
interstitium.
Second, the descending limb, which is in very close proximity to the ascending
limb, is solute-impermeable and water-permeable. Because the ascending and
descending limb share the same interstitial fluid, water moves along the osmotic
gradient from the descending limb into the interstium, concentrating the tubular
filtrate in the descending limb. Thus, the osmolarity of the medullary
interstitial fluid along this limb steadily increases, and water leaves the
filtrate. The osmolarity of the filtrate is highest (1,200 mOsm) where the
nephron loop bends, and the filtrate moves from the descending limb to the
ascending limb.
The third factor in the countercurrent multiplication is the shift of the fluid
along the length of the tubule so that fluid that participated in water loss in
the descending limb, will now participate in solute loss in the ascending limb.
When the filtrate in the descending limb turns the corner and enters the
ascending limb, the concentrations of sodium and chloride ions are very high in
the filtrate The molecules are now actively pumped from the tubule into the
interstitial fluid to maintain the high osmotic pressure in the interstitium. It
turns out that this system “traps” high salt concentrations deep in the medulla
as the ions are most actively pumped out there.
Losing salt but not water makes the filtrate in the ascending limb increasingly
more dilute. By the time it reaches the distal tubule, it is at 100 mOsm and
therefore has a lower osmotic pressure than the blood plasma and interstitial
fluids in the renal cortex. This is what allows for the generation of a dilute
urine.
Another contributor to the high osmolarity of the the medullary interstitium is
the recycling of urea. Urea from the interstitial fluid diffuses into the
filtrate in the thin limbs of the nephron loop. Because the thick limb and
collecting duct are urea-impermeable, by the time the filtrate reaches the
collecting duct, water reabsorption has created highly concentrated urea that
diffuses back into the medullary interstitial fluid. The resulting pool of
urea is a major contributor to the high osmolarity in this region. This urea is
then recycled back into the thin limbs of the loop, and the cycle starts again.
The presence of ADH amplifies urea recycling, which, in turn, amplifies the
osmotic gradient and enables the formation of more concentrated urine.
Urine Transport
The difference in hydrostatic pressure between the glomerular capsule (10 mm Hg)
and the renal pelvis (practically no pressure at all) creates a pressure
gradient that forces filtrate to flow from the glomerular capsule through the
tubules and into the renal pelvis. In contrast, no pressure gradient exists to
propel the flow of urine through the ureters and into the urinary bladder.
Instead, urine flow at this point is controlled by peristaltic contractions in
the circular smooth muscle of the ureter walls. These peristaltic waves vary in
frequency from once every few seconds to once every two to three minutes. Their
frequency is increased by parasympathetic stimulation and decreased by
sympathetic stimulation.
Example
Kidney Stones
Kidney stones are one of the most painful urinary system disorders. Also called
calculi ("little stones"), kidney stones are not a new disorder. Scientists have
discovered them in 7,000-year-old Egyptian mummies. Kidney stones are formed when
the uric acid salts, magnesium, or calcium in urine crystallize. Certain types of
kidney stones run in families and appear to have a hereditary component. Some
substances in foods may also contribute to the incidence of kidney stones. Most
calculi are small enough to pass through the urinary tract and be eliminated with
urine. Stones larger than five millimeters in diameter, however, can prevent urine
from draining. The backed-up urine exerts increasing pressure in the kidney, causing
extreme pain in the back and side near the kidney or in the lower abdomen.
Dehydration can also contribute to the formation of kidney stones. Each year, about
2.4 million Americans seek treatment for kidney stones. Kidney stones are more
common in people with a family history of calculi, or who have urine retention or
frequent bacterial urinary tract infections. The most commonly used treatment for
kidney stones is a procedure called extracorporeal shock wave lithotripsy. Shock
waves created outside the body pass through the skin and body tissues and break down
the stones into small particles that can be eliminated in the urine. In severe
cases, endoscopic or open surgery may be required to remove the stones.
Urine Storage
Much more time is spent storing urine than micturating (urinating). When urine
accumulates in the bladder, distension of the bladder walls activate stretch
receptors. These receptors transmit signals through visceral afferent fibers to
the sacral area of the spinal cord. The signals initiate spinal storage
reflexes, which enhance sympathetic inhibition of the bladder's
detrusor muscle and maintain the internal sphincter in a closed position. The
reflexes also stimulate pudendal motor fibers, which cause the external urethral
sphincter to contract, preventing the urine from escaping.
Urine Elimination
As previously noted, micturition (also called urination or voiding) is the
process of emptying urine from the bladder and is the result of involuntary and
voluntary muscle contractions. When the amount of urine accumulated in the
bladder reaches about 200 milliliters (7 ounces) (though this amount varies from
person to person), afferent impulses are sent to the sacral region of the spinal
cord that initiate a reflexive relaxation of the internal urethral sphincter and
contraction of the detrussor muscle. The afferent signals that stimulate the
urge to urinate are also sent to the brain. This allows the person to relax
their external uretheral sphincter, made of skeletal muscle, so that micturition
will occur. If we do not void immediately, the reflexive responses will
initially weaken, but as more volume is added to the bladder, these responses
will come back more strongly, creating a more urgent need to void.
When we are ready to empty the bladder—a decision executed by the cerebral
cortex—the micturition reflex is set in motion. Afferent impulses
activate the micturition center of the brain. This signal
intregrates with parasympathetic signals of the spinal cord to allow the
external sphincter to relax, thus releasing urine from the bladder.
If we need to delay micturiition, the reflex bladder contractions will taper off
and stop within about one minute, and urine will continue accumulating. The
addition of another 200 to 300 milliliters (7 to 10 ounces) of urine will prompt
another micturition reflex. If voiding is still not possible, the reflexes will
again subside. When more than 500 to 600 milliliters (about 20 ounces) of urine
accumulates, urination will occur whether we want to or not. After micturition,
about 10 milliliters (0.33 ounces) of urine will remain in the bladder.
Let’s summarize
Nephrons and collecting ducts carry out three basic functions in urine
production:
-
glomerular filtration where water and solutes in blood plasma pass through
the wall of glomerular capillaries and filter into the renal tubules about
one fifth of the plasma that flows through the kidneys.
-
tubular reabsorption where cells of the tubule reabsorb almost all water
some solutes from the filtrate as it flows through the renal tubule and
collecting duct. These reabsorbed substances are then returned to the blood
via the peritubular capillaries and vasa recta.
-
tubular secretion where cells in the renal tubule and collecting duct
secrete waste from the blood as it flows through the tubule and duct.
Three forces affect the rate of glomerular filtration; one enhances filtration,
and two resist it. Subtracting the opposing pressures from the promoting
pressure yields the net filtration pressure.
The glomerular filtration rate (GFR) is the total amount of filtrate formed by
the two million renal corpuscles in the kidneys divided by time.
- The average GFR is 125 milliliters (4 ounces) per minute, which adds up to
nearly 140 liters (50 gallons) a day.
- The kidneys must maintain a relatively consistent GFR to maintain
homeostatic balance in body fluids.
Reclaimed fluid that is sent back into the bloodstream to maintain water balance
and ion concentrations occurs through reabsorption in the tubules. Reabsorption
returns about 99 percent of filtered water and many filtered solutes to the
bloodstream.
- Each day, about 180 liters (190 quarts) of water are filtered in the
kidneys. Only 1–2 liters (1–2 quarts) are excreted in urine; the rest is
reabsorbed in the kidneys.
Reabsorption is performed by cells in the proximal convoluted tubule, epithelial
cells all along the length of the renal tubule, and the collecting duct.
The majority of solute and water reabsorption occurs in the proximal convoluted
tubule and sodium ions participate in most solute reabsorption.
Secretion removes the additional solutes from the plasma and actively transports
them to the tubule.
Tubular secretion occurs mostly in the proximal convoluted tubule and removes
unwanted substances from filtrate by transferring unwanted substances from the
blood and tubule cells into the tubular fluid.
Tubular secretion is important because:
- It removes substances that are not easily filtered
- It disposes of unwanted substances or end products, such as urea, that have
been passively reabsorbed.
- It eliminates excess potassium ions.
- It regulates blood pH.
In the urinary system, osmosis plays a major role in the regulation of urine
concentration.
Osmotic activity is the ability of the solution to cause osmosis and depends on
the amount of solute particles that cannot pass through the semipermeable
membrane.
Osmolarity is defined as the number of solute particles dissolved in one kilogram
of water. The osmotic activity of a solution is based on the osmolarity of that
solution.
Formation of Dilute and Concentrated Urine:
- Dilute urine is produced when the pituitary gland is not secreting the
antidiuretic hormone (ADH) and the diluted filtrate goes to the renal
pelvis.
- When water conservation is required, the kidneys will produce a small amount
of concentrated urine to preserve water, while still eliminating wastes and
surplus ions. ADH inhibits urine production.
Micturition is the process of urination
- Which begins with blood carrying various wastes enters each of the two
kidneys through the renal artery.
- From there, afferent impulses are sent to the sacral region of the spinal
cord when the amount of urine in the bladder reaches about 200 milliliters.
- Once afferent impulses are perceived, a reflexive relaxation of the internal
urethral sphincter is initiated and contraction of the detrussor muscle
occurs.
- When more than 500 to 600 milliliters (about 20 ounces) of urine accumulate
in the bladder, involuntary urination will occur.
- Micturition ends when urine exits out of the body through the urethra.
- After micturition, about 10 milliliters (0.33 ounces) of urine will remain
in the bladder.
The primary function of the urinary system is to maintain homeostasis of fluid and small
molecules within the body. We will start with a molecular characterization of urine
followed by molecular and tissue level descriptions of the structures that are
responsible for the formation of the urine. Then we will follow the movement of material
from the bloodstream, through the functional units of the kidney through the other
organs of the urinary system, and out of the body.
Molecular level – The Components of Urine
Our input of water varies greatly from day to day. Despite major differences in
fluid intake, the total volume of fluid within the body remains the
relatively constant. The body’s fluid homeostasis is achieved largely due to the kidneys'
ability to regulate how much water is excreted in urine. If a person with healthy
kidneys drinks a large volume of fluids, the kidneys will produce a large volume
of urine. When a person with healthy kidneys does not drink enough fluids or
experiences significant fluid loss, the kidneys will produce a small amount of
concentrated urine to conserve water.
Urine is composed of urea, chloride, sodium, potassium, creatinine and other
compounds (ions, inorganic and organic). Urea is an organic breakdown product of
nitrogenous materials.
The Clinical Characteristics of Normal Urine
| Characteristic |
Normal Value/Nature |
| Color/transparency |
Yellow/clear |
| Odor |
Varies from slightly aromatic to ammonia-like; food and beverages can
change the odor |
| pH |
4.5 to 8.0 (average: 6.0) |
| Specific gravity (the density of urine compared with the density of pure
water) |
1.001 to 1.035 |
| Water content |
95 to 97 percent |
| Volume |
1 to 2 liters/ day (quarts/day) |
The functional units of the kidneys are called nephrons. We will cover the
anatomy and physiology of the nephrons in the next section. To understand how
different regions of the nephron are able to have unique, spatial functions, we
will first discuss how the multicellular epithelial structures found in these
structures are organized for specific functions.
Nephrons are made up of epithelial cells with an underlying non-cellular layer or
basement membrane that separates the filter in the lumen fluid from the
interstitial space. These epithelial cells differ in cellular anatomy along the
length of the nephrons according to the filtering and processing functions of
the epithelial cells.
Functional Urinary Tissue – The Nephron
As the chief functional organ in the urinary system, the kidneys excrete
nitrogenous wastes and are involved in regulating the volume, composition, and
pH of the blood. The kidneys receive one fourth of total cardiac output, a
reflection of their function as blood processors. Each kidney contains about one
million nephrons, the structural and functional units of the kidneys. Each
nephron is made up of a high-pressure capillary bed called a glomerulus and a
renal tubule, segments of which included a proximal convoluted tubule, nephron
loop (loop of Henle), and distal convoluted tubule. The distal convoluted
tubules from multiple nephrons join a common collecting duct. The nephrons are
involved in three functions: filtration, reabsorption, and secretion.
Overview of the Nephron Structure
The structure within each nephron that actually filters blood plasma is
the renal corpuscle containing the glomerulus and
glomerular capsule. Another nephron structure called the renal
tubule receives the filtered fluid, called glomerular
filtrate. Very thin and a little over an inch long, the renal
tubule has three major consecutive segments that the filtrate flows
through: a proximal convoluted tubule the nephron
loop (loop of Henle), and a distal
convoluted tubule.
Nephron Functions
| Nephron Structure |
Function |
Description |
| Glomerulus |
Filtration |
The glomerulus is a capillary network found in close proximity
to the nephron that filters plasma into the nephron.Proteins and
blood cells are retained in the glomerular capillary. |
| Tubules and nephron loop (loop of Henle) |
Reabsorption |
Epithelial cells actively transport some substances from the
tubular fluid back into blood. Other substances, such as water,
are passively reabsorbed in some segments. |
| Capillaries specifically Peritubular |
Secretion |
Epithelial cells actively secrete certain substances from the
blood into the tubular lumen. |
| Collecting duct |
Collection |
Accumulates any material that is not returned to blood in the
preceding segments. Secretes or reabsorbs H+, HCO3+,
and K+ ions. Reabsorbs water under the influence of
anti-diuretic hormone. Anything left in the distal end of the
collecting duct will l be excreted as urine |
The Glomerulus
The renal corpuscle is made up of a tangled capillary network
called a glomerulus and a cup-shaped structure
called the glomerular capsule (Bowman's
capsule) surrounding the glomerulus. The glomerular
capsule has an external parietal layer made of simple squamous
epithelium. Although this layer is not involved in the
production of filtrate, it helps to maintain the shape of the
capsule. An inner visceral layer adheres to the glomerular
capillaries and is composed of a special type of simple squamous
epithelial cells called podocytes. These podocytes
have multiple projections called pedicels or foot
processes. The pedicels of one podocyte interlock
with the pedicels of adjacent podocytes. Filtrate from the
glomerulus passes through filtration slits, the openings between
the foot pedicels, to enter the capsular space
(Bowman's space), the area between the visceral
and parietal layers of the glomerular capsule.
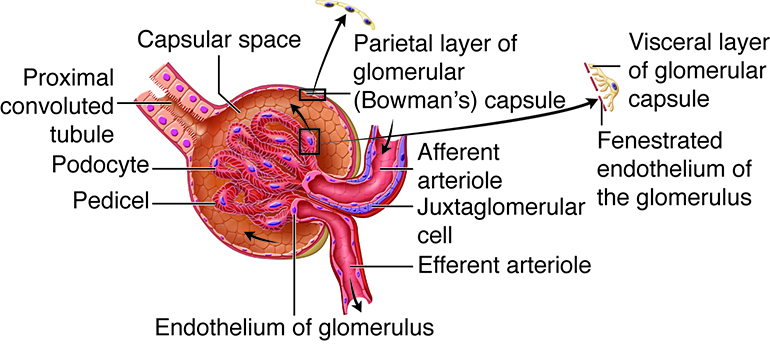 Detail of glomerulus. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States
(http://creativecommons.org/licenses/by/3.0/us/).
Detail of glomerulus. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States
(http://creativecommons.org/licenses/by/3.0/us/).
The Proximal Convoluted Tubule
The proximal convoluted tubule (convoluted
refers to the coiled shape) tubule connects the glomerular
capsule to the nephron loop. The apical surface of the simple
cuboidal epithelial cells making up the proximal convoluted
tubule are covered in microvilli producing a brush border. The
brush border and the length of the proximal convoluted tubule
dramatically increase the luminal surface area available for
reabsorbing water and solutes and for secreting substances into
the filtrate.
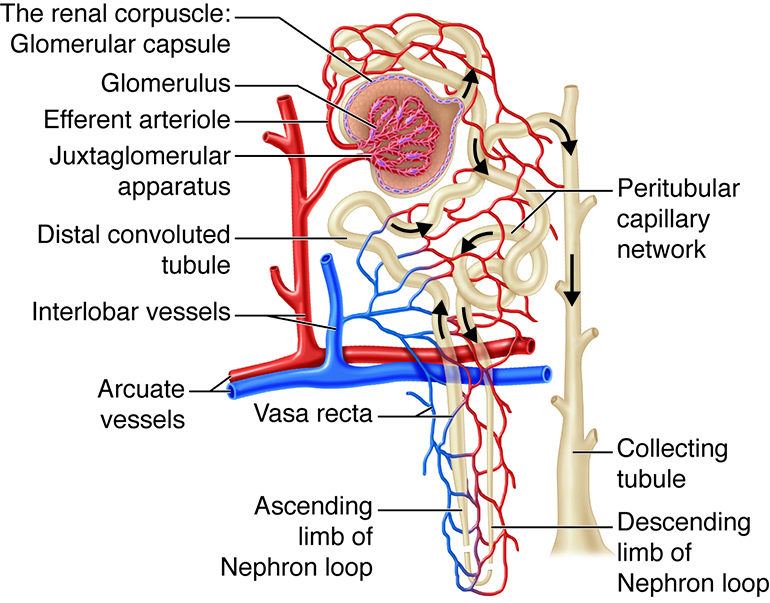 Tubules. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Tubules. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The Nephron Loop
The renal corpuscle, the proximal convoluted tubule, and the
juncture between the proximal convoluted tubule and the nephron
loop are located in the renal cortex. The first part of the
nephron loop, the descending limb of the nephron loop, drops
into the renal medulla. In the renal medulla, the loop makes a
sharp, almost 180-degree turn back toward the renal cortex as
the ascending limb of the nephron loop.
The ascending limb is continuous with the distal convoluted
tubule. The ascending and descending limbs of the nephron loop
have two distinct parts: a thin section of the limb and a thick
section of the limb. In the thin section of the limb, the
diameter of the tubule is distinctly smaller than the diameter
of the rest of the nephron tubules. In the thin sections of the
limbs, the epithelium is thinner simple squamous epithelium that
is permeable to water. In the thick sections of the limbs, the
epithelium is simple cuboidal epithelium that is highly
impermeable to water. Regardless of being in the thin or the
thick segments, the lumen is the same size as the lumen in the
rest of the renal tubule.
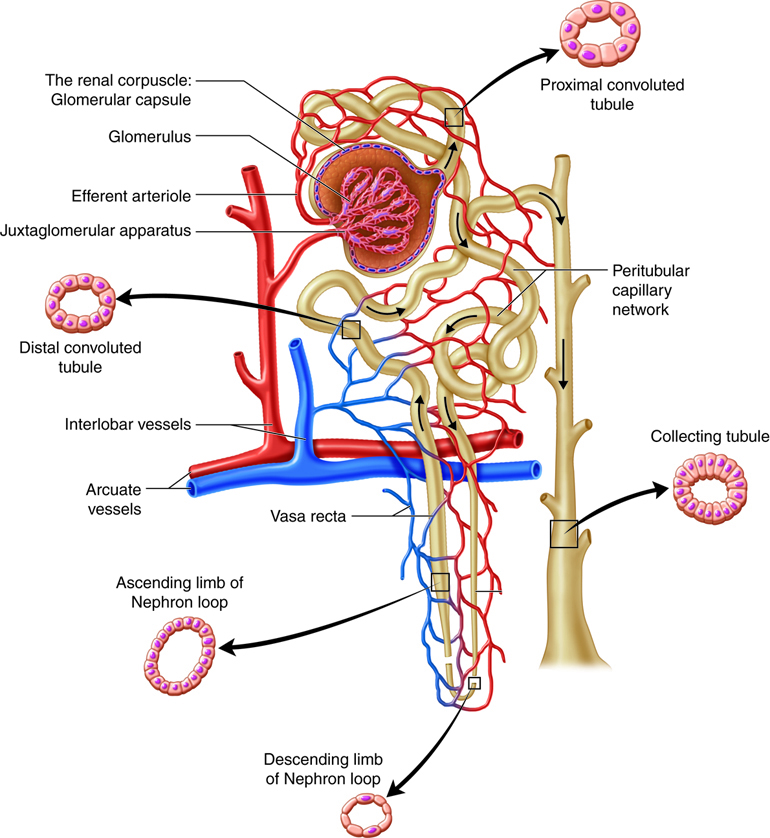 Loop of Henle. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Loop of Henle. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Distal Convoluted Tubule
The final segment of the nephron is the distal convoluted tubule.
As the ascending limb of the nephron loop reaches the renal
cortex, it becomes the distal convoluted tubule. The distal
convoluted tubule extends to the collecting tubule, the short
connection with a collecting duct. The distal convoluted tubule
is composed of simple cuboidal epithelium with very few
microvilli and no brush border.
Capillaries of the Nephron
The glomerulus is not the only capillary bed associated with
nephrons. Peritubular capillaries are branches of
the efferent arterioles that drain the glomeruli
and recover most of the filtrate produced in the renal
corpuscle. Glomerular capillaries differ from other capillary
beds in the body, because they are both supplied by and drained
by arterioles. The feeder afferent arterioles are
branches of the cortical radiate arteries. The draining efferent
arterioles branch into the peritubular capillary network around
the proximal and distal convoluted tubules or the vasa recta
around the nephron loop. The diameter of the draining efferent
arterioles is smaller than that of the afferent arterioles,
giving the efferent arterioles higher resistance. Because of
this, the glomerulus has a high blood pressure that allows it to
filter high volumes of fluid and solutes out of the blood and
into the glomerular capsule. The nephrons segments reabsorb
approximately 99 percent of this filtrate. The peritubular
capillaries adhere to neighboring convoluted tubules and drain
into neighboring venules. These low-pressure and porous
capillaries easily reabsorb the water and solutes that the
tubule recovers from the filtrate. In some nephrons, rather than
breaking up into peritubular capillaries, the efferent
arterioles form clusters of thin-walled vasa recta. Important
for the formation of concentrated urine, the vasa recta
are long, straight capillaries that reach deep into the
medulla alongside the longest nephron loops where they can
collect reabsorbed substances from the loop segments.
Because blood in the renal circulation flows through two
arterioles (where a majority of the manipulation of vascular
resistance is found), renal blood pressure drops from about 95
mm Hg in the renal arteries to less than 10 mm Hg in the renal
veins. Resistance in the afferent arterioles fluctuates in order
to help maintain a relatively constant glomerular capillary
hydrostatic pressure even if there are substantial changes in
systemic blood pressure. The resistance of the efferent
arterioles also contributes to maintenance of glomerular
capillary hydrostatic pressure and also contributes to a low
hydrostatic pressure in the peritubular capillaries.
Specialized Cells Associated With the Nephron
In all nephrons, the last part of the ascending limb of the
nephron loop transitions into the distal convoluted tubule and
comes in contact with the afferent arteriole supplying the renal
corpuscle. In this region the columnar epithelial cells at the
beginning of the distal convoluted tubule are very crowded,
leading to the name macula densa ("dense spot").
The macula densa is believed to monitor sodium chloride
concentration of the filtrate entering the distal convoluted
tubule. The wall of the afferent arteriole that is adjacent to
the macula densa contains granular cells (also known as
juxtaglomerular cells ). The granular cells
produce and secrete the enzyme renin and are also capable of
contracting when stimulated. These cells and the macular densa
make up the juxtaglomerular apparatus. The action
of the juxtaglomerular apparatus helps control glomerular
hydrostatic pressure by sending signals to the afferent
arteriole. There are also special smooth muscle cells called
intraglomerular mesangial cells in in the spaces between the
loops of the glomerulus. These cells help regulate blood flow
through the glomerulus.
Collecting Ducts
As the functional units of the kidneys, the primary role of the
nephrons is to filter plasma, reabsorb what the body would like
to keep, and excrete the rest. Any substances not reabsorbed
in the tubules of the nephrons flows into one of thousands
of collecting ducts in the kidney. A short collecting tubule
forms the juncture between a distal convoluted tubule and a
collecting duct. Each collecting duct receives fluid from
several nephrons and then transports it to the renal pelvis. The
collecting tubules and ducts have two types of cells:
intercalated cells, cuboidal cells with plentiful microvilli,
and the more populous principal cells, cuboidal cells with
limited, short microvilli. Principal cells help maintain water
and sodium and potassium ion balance in the body. Intercalated
cells help regulate the acid-base balance of the blood.
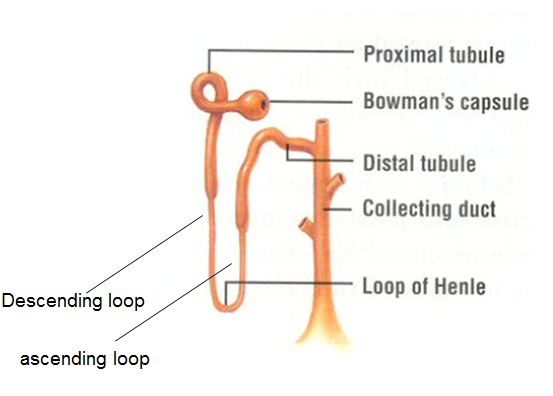 Collecting ducts. This work by Cenveo is licensed under
a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Collecting ducts. This work by Cenveo is licensed under
a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
There are six organs in the urinary system: two kidneys, two ureters, the urinary
bladder, and the urethra.The kidneys are the body's main purification
system. They remove wastes, some of which are toxic, from the blood. The kidneys also
help to regulate blood composition and volume. By manipulating blood volume, the kidneys
contribute to the regulation of blood pressure. The kidneys are about the size of your
fist and are shaped like beans. The nephron, which we have already covered,
is the structural and functional unit of the kidney. The average number of nephrons in
each kidney is about one million, although this number can vary from about half a
million to two million. Nephrons are so good at what they do that it only takes about
one fourth of them to be functional to meet the needs of the body. Still, a low number
of functioning nephrons has been linked to a greater risk of developing kidney disease
and high blood pressure (hypertension).
Kidney Location
The kidneys lie between the parietal peritoneum and the posterior abdominal wall,
just above waist level. The location posterior to the parietal peritoneum means
they are retroperitoneal. These reddish, paired organs are offered
some protection by the eleventh and twelfth rib pairs. The right kidney lies
slightly lower than the left kidney. This asymmetry occurs due to the increases
space required by the liver on the right side of the abdominal cavity superior
to the kidney.
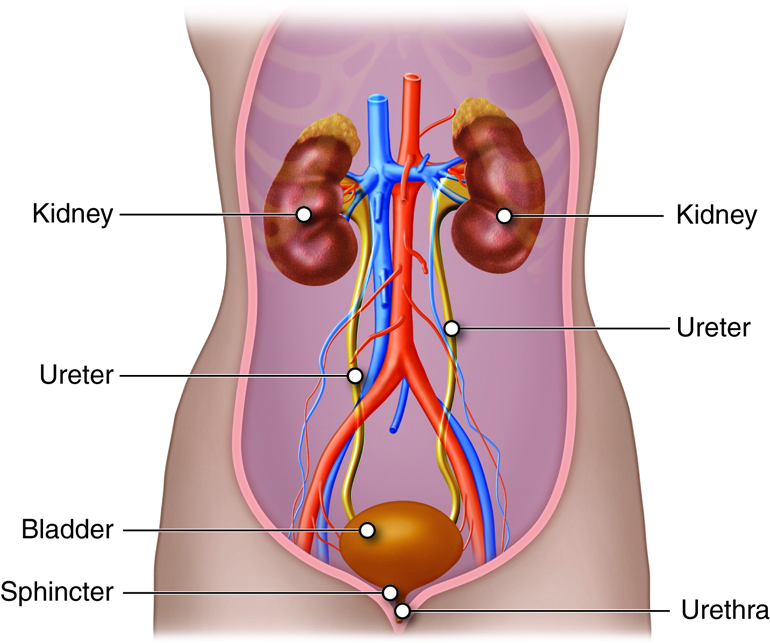 Inside look at organs. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Inside look at organs. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
External Anatomy
In adults, the kidney is approximately four to five inches long, two to
three inches wide, and one inch thick (10 cm long, 5 cm wide and 2 cm
thick). On average, each kidney weighs just under five ounces. The
kidneys have a concave medial border that faces the vertebral column.
There is a depression near the middle of the concave border called the
renal hilum, where the renal artery enters the kidney
and the renal vein, and ureters leave it.
Each kidney is enshrouded in four tissue layers. The innermost layer, the
renal capsule, is composed of fibrous connective
tissue. It preserves the form of the kidney while protecting it from
damage due to trauma. The adipose capsule or
perinephric fat is the middle tissue layer. This fatty
tissue mass encircles the renal capsule, offering another layer of
protection from trauma and fixing the kidney in place. The renal
fascia is the third tissue layer. The renal fascia is a slim
layer of dense irregular connective tissue. This layer tethers each
kidney to neighboring structures and to the abdominal wall. The
paranephric fat forms the superficial layerand provides additional
cushion and support for the kidney.
 Cross section of kidney. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/). Cross section of kidney. This work by Cenveo is licensed
under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
|
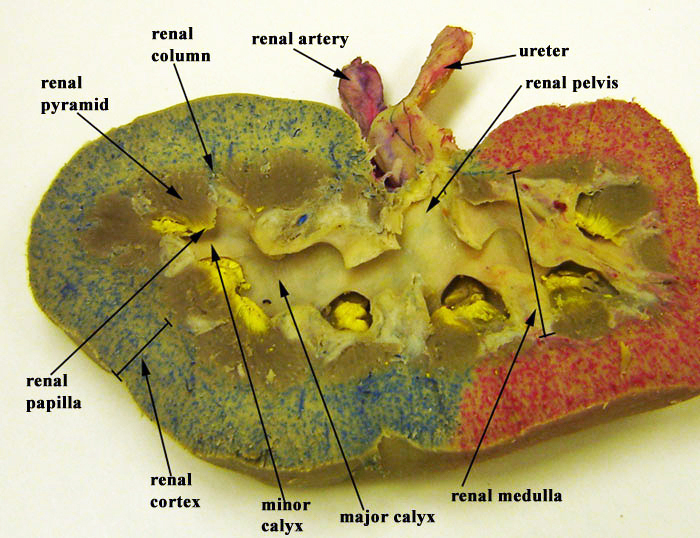 Cross section of kidney. Image courtesy of Deborah Y. Sillman, Penn State University. Cross section of kidney. Image courtesy of Deborah Y. Sillman, Penn State University.
|
Example
Renal Ptosis
The position of the kidneys is in part maintained by the layers of fat
surrounding them. People who lose a lot of weight in a short period of time
can also lose some of the fat that surrounds their kidneys. Very thin people
may not have a sufficient layer of fat around their kidneys. In either case,
one or both kidneys may drop down into the pelvis when a person stands up, a
condition called renal ptosis (ptosis:
"to fall"). For the majority of cases, people are without symptoms. However,
in some cases health problems ranging from acute pain and vomiting to
kinking of the ureter can occur.
If renal ptosis creates a kink in the ureter, the kink can prevent the
drainage of urine. Urine can then back up into the kidney, creating pressure
that may damage renal tissue.
Diagnosis is controversial but may be confirmed by a patient experiencing
relief from abdominal pain upon lying in a supine position and/or by imaging
tests.
Treatment for severely symptomatic patients usually involves a laproscopic
surgical procedure called nephropexy. This procedure affixes
the kidney to the retroperitoneal tissue, closer to its usual position.
Internal Anatomy
Two structures dominate the internal anatomy of the kidney: a deep
reddish-brown area called the renal medulla, and a
superficial pinkish area called the renal cortex. The renal
medulla is made up of cone-shaped structures called renal
pyramids and its primary purpose is to maintain the proper
balance of salt and water in the blood. The bases of the pyramids border
the renal cortex, and their apexes (renal papillae) face
the renal hilum. The smooth-textured renal cortex runs from the renal
capsule to the renal pyramid bases and extends towards the pelvis in the
spaces between the pyramids. The renal cortex has an outer cortical zone
and an inner juxtamedullary zone. The areas of renal cortex lying
between renal pyramids are called renal columns. A
renal lobe consists of one renal pyramid with its
surrounding renal cortex, including one half of both adjacent renal
columns.
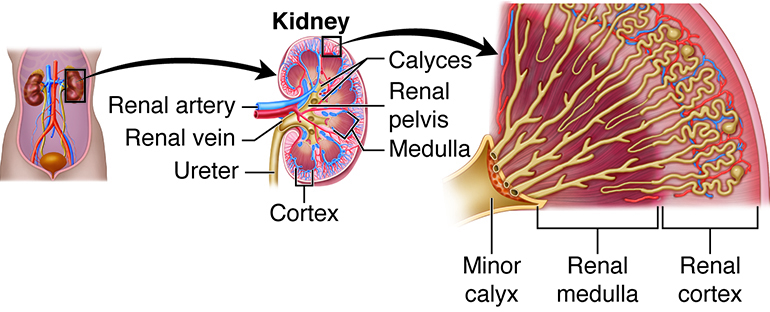 Internal structure of kidney. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Internal structure of kidney. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The parenchyma refers to the functional part of any organ.
In the case of the kidney, the parenchyma includes the renal cortex and
the renal pyramids. The actual functional units of the kidneys are
microscopic structures called nephrons. Recall that there are about a
million of nephrons per kidney. A low nephron number is associated with
an increased risk of kidney disease and high blood pressure
(hypertension).
One of the nephrons' main roles is to create urine. Urine produced by
nephrons empties into large papillary ducts. These ducts
run through the renal papillae of the pyramids. From the papillary
ducts, urine flows into cup-like structures called the minor and
major calyces (calyces: "cups"). Each
kidney contains two or three major calyces and several minor calyces.
The papillary ducts of one renal papilla drains into a minor calyx. As
minor calyces join together, they form a major calyx. All major calyces
join together to form one large chamber called the renal
pelvis. Urine that collects in the renal pelvis is
transported out of the pelvis through the ureters and to the urinary
bladder.
Within the kidney, the hilum opens up into a cavity called the
renal sinus, which includes a portion of the renal
pelvis, the calyces, and renal blood vessel and nerve branches. These
structures are held in place in the renal sinus by adipose tissue.
Blood and Nerve Supply
The kidneys' generous supply of blood vessels reflects their roles in the
removal of wastes from the plasma and regulators of the volume and ionic
composition of blood. Despite the kidneys accounting for less than 0.5
percent of total body mass, the right and left renal arteries
transport approximately one fourth of total cardiac output
(approximately1.2 quarts or 1 liter) to these organs every minute.
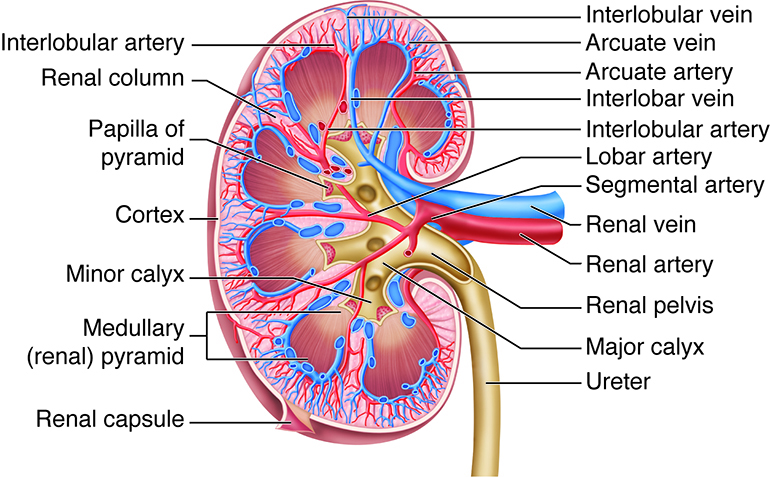 Cross section of kidney arteries and veins. This work by Cenveo is
licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Cross section of kidney arteries and veins. This work by Cenveo is
licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The large renal artery divides into several segmental arteries
within the kidney that supply different areas or segments of the
kidney. The segmental arteries branch into a number of interlobar
arteries within the renal column. At the corticomedullary
junction, the interlobar arteries branch to form the arcuate
arteries (arcuate means curved). The arcuate arteries divide
into smaller cortical radiate arteries (also called
interlobular arteries) that supply the cortical tissue.
The cortical radiate arteries divide into afferent arterioles, which
supply nephrons. The renal cortex receives more than 90 percent of the
renal blood supply.
Veins generally follow the same courses as arteries, but in reverse. From
the renal cortex, blood drains first into the cortical radiate
(interlobular) veins and then the arcuate, interlobar, and renal veins;
there are no segmental veins. The renal veins drain into the inferior
vena cava, which is located to the right of the vertebral column.
Because of the position of the inferior vena cava, the left renal vein
is about twice as long as the right renal vein.
Innervation of the kidneys and their ureters is supplied from an
outgrowth of the celiac plexus called the renal plexus.
This complex of autonomic nerve fibers and ganglia is primarily supplied
by sympathetic vasomotor fibers. Their motor function is to adjust the
diameter of renal arterioles to help regulate renal blood flow. This
includes regulation of blood flow in the affrent and efferent arterioles
and thus in the glomerulus. These fibers also innervate the juxtaglomerular apparatus.
The Ureters
The ureters are a pair of thin, muscular tubes that transport urine
from the kidneys to the bladder. Beginning at the level of the second lumbar
vertebra, the location of the ureters is retroperitoneal. Each ureter runs
inferiorly and enters the posterolateral wall of the urinary bladder. This angle
of entry is important, because it helps prevent urine from flowing back into the
ureters when the bladder fills with urine. In addition, accumulating urine
increases the internal pressure of the bladder, and this pressure compresses and
seals the distal portion of the ureters.
There are three layers in the ureter wall. The innermost mucosa lining contains
transitional epithelium capable of stretching but is impermeable to urine. The
ability to stretch allows the ureter wall to accommodate changing volumes of
urine. The middle muscularis layer is composed of two layers of smooth muscle:
an inner longitudinal layer and outer circular layer. In the lower third of the
ureter, the muscularis has a third outer layer of longitudinal muscle fibers.
The muscularis layer is responsible for the peristaltic contractions needed to
move urine through the ureters and into the bladder. The external layer of the
ureter wall, the adventitia, is made of fibrous connective tissue and helps
anchor the ureter to the abdominal wall.
When urine enters and distends the ureters, stretch receptors are stimulated.
Reflexive action results in the contraction of the muscularis and, movement of
the urine into the bladder. The power and frequency of peristalsis is directly
related to the rate of urine formation. Although the ureters are innervated by
both sympathetic and parasympathetic fibers, the nervous system does not appear
to have major involvement in the transport of urine in these organs.
The Urinary Bladder
The urinary bladder is a hollow, collapsible muscular sac that
serves as a temporary storage facility for urine. It is located in the pelvic
cavity, just posterior to the pubic symphysis. In females, the bladder lies
anterior to the vagina and inferior to the uterus. In males, it is immediately
anterior to the rectum. Peritoneal folds hold the bladder in
place.
The bladder can hold up to about a liter of urine, although this amount varies
from person to person. Despite its capacity to enlarge, an overfull bladder can
burst but it is more likely that excess urine will leak out of the urethra. When
empty, the bladder collapses into a pyramidal shape. When a small amount of
urine accumulates, it is spherical. When a larger volume of urine accumulates,
the bladder becomes pear-shaped and ascends in the abdominal cavity. There are
three openings in the bladder: two for the ureters and one for the urethra.
These openings frame a triangular region at the base of the bladder called the
trigone.
The bladder wall is made up of a mucosa with transitional epithelium, a
submucosa, a thick muscularis called the detrusor muscle, and a
fibrous adventitia. The adventitia is on the inferior surface only. In contrast,
the peritoneum covers the superior surface. The detrusor muscle is composed of
inner and outer layers of longitudinal smooth muscle fibers and an intermediate
layer of circular muscle fibers.
The Urethra
The urethra is a small muscular tube that transports urine from the
bladder out of the body. The urethra is five times longer in males (8 inches, 20
cm) than in females (1.6 inches, 4 cm). In males, the urethra is also part of
the reproductive system, providing a passageway for semen as well as urine. The
course of the urethra also differs between the sexes. In females, fibrous
connective tissue binds it to the anterior vaginal wall, and its external
urethral orifice (external opening) is located anterior to the
vaginal opening and posterior to the clitoris. In males, the urethra is divided
into three regions. The prostatic urethra is surrounded by the
prostate. The membranous urethra passes through the urogenital
diaphragm. The spongy urethra runs through the penis and ends at
the external urethral orifice.
At the bladder-urethra junction, the circular fibers of the bladder's detrusor
muscle form the internal urethral sphincter. When urine is not
draining from the bladder, this involuntary sphincter closes off the urethra to
prevent the leakage of urine when you are not voiding. At the point where the
urethra passes through the urogenital diaphragm, it is surrounded by the
external urethral sphincter, a skeletal muscle. The external
sphincter is voluntarily controlled and is kept contracted until voiding.
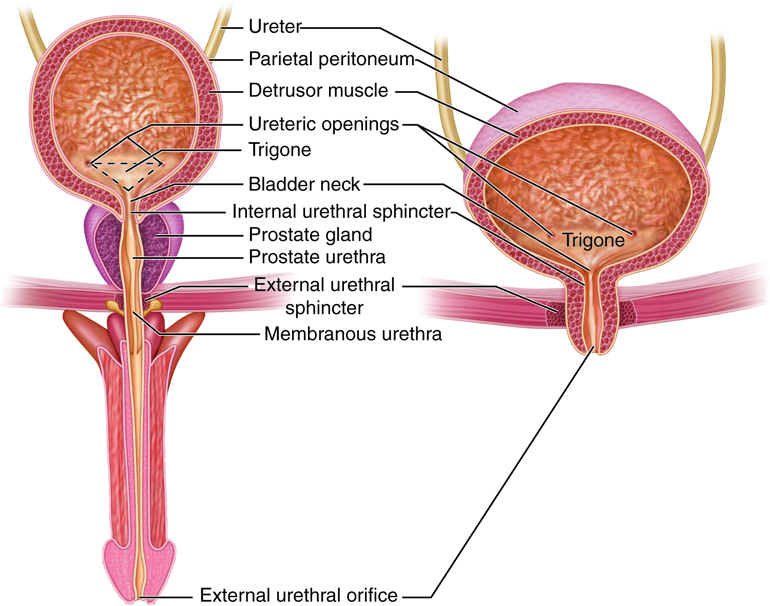 Urethra. This work by Cenveo is licensed under a Creative Commons
Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Urethra. This work by Cenveo is licensed under a Creative Commons
Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Example
Urinary Tract Infections
Urinary tract infections (UTIs) are the most common type of bacterial infection.
Women are predisposed to UTIs because their urethras are shorter than those of men.
Moreover, the urethra's external opening in women is closer to the anus than it is
in men. Over 50 percent of women will have a UTI at some point during their
lifetime. Fecal bacteria such as Escherichia coli (E. coli) can easily travel up the urethra. This is why women should never
wipe the anus in a forward direction after defecation. However, most UTIs in women
occur as a result of sexual activity. During intercourse, bacteria from the external
genital area and the vagina can be pushed up the short urethra toward the bladder.
The use of spermicides actually increases the risk of UTIs, because spermicides kill
bacteria that would otherwise help destroy infectious fecal bacteria in the vagina.
Drinking plenty of water and urinating immediately after sexual activity can help
prevent UTIs by flushing bacteria out of the urethra. Infection of the urethra
(urethritis) can easily spread to the urinary bladder
(cystitis) and sometimes to the kidneys (pyelitis or
pyelonephritis). Symptoms of a UTI include pain during urination
(dysuria), frequent urination or an urgent need to urinate,
cloudiness or blood in the urine (hematuria), urine with a strong odor,
nausea, and fever. Fortunately, most UTIs respond to antibiotics. Analgesics may
also be prescribed to reduce discomfort. Unfortunately, having a UTI increases the
chances of having subsequent UTIs. UTIs are also common in infants, particularly in
uncircumcised male infants.
Let’s summarize
Despite major differences in fluid intake on a given day, the total volume of
fluid within the body usually remains the same. This is due to homeostasis. For example, if a person with healthy kidneys drinks a
large volume of fluids, the kidneys will produce a large volume of urine.
Conversely, if that same person does not drink enough fluids, the kidneys will
produce a small amount of concentrated urine to preserve water.
Urine is composed of elements and compounds that include urea, chloride, sodium,
potassium, creatinine, hydrogen, and bicarbonate. The nephrons help produce
urine.
The functional units of the kidneys are called nephrons,
which are made up of epithelial cells sitting on top of a non-cellular layer or
basement membrane.
Each kidney contains about one million nephrons, which are made up of:
-
A renal corpuscle made up of a glomerulus within a glomerular capsule
-
A renal tubule comprised of a proximal convoluted
tubule, nephron loop, and distal convoluted tubule.
-
Collecting ducts that receive tubular filtrate from
multiple nephrons and transport the fluid to the calyces and renal
pelvis.
The nephrons are involved in three functions: filtration, reabsorption, and
secretion.
There are six organs in the urinary system: two kidneys, two ureters, the urinary
bladder, and the urethra.
The two kidneys, perform the following functions:
-
Remove wastes from the blood.
-
Help regulate blood composition and volume.
- Contributing to the regulation of blood pressure.
External Anatomy of the kidneys include:
- A renal hilum, renal vein, ureter, and nerves either
enter or exit the kidney.
- A renal capsule that preserves the form of the
kidney.
- The perinephric fat that encircles the renal capsule,
offering another layer of protection.
- The renal fascia that tethers each kidney to
neighboring structures and to the abdominal wall.
- The paranephric fat that comprises the outermost
layer, and provides another layer of protection.
Internal Anatomy of the kidneys include:
- Therenal medulla that maintains the proper balance of
salt and water in the blood
- The renal cortex is the outer layer of the kidney. It
also extends as renal columns between the renal pyramids.
- The renal pyramids make up the renal medulla Included
as part of the functional part of the kidney.
- The renal papillae are located at the apex of the
renal pyramids.
- The renal columns are part of the renal lobes.
- The renal lobes consist of one renal pyramid with its
surrounding renal cortex, including one half of both adjacent renal columns.
The other organs of the urinary system include:
The ureters:
- The innermost mucosa lining allows the ureter wall to
accommodate changing volumes of urine.
- The middle muscularis layer is composed of two layers
of smooth muscle: an inner longitudinal layer and outer circular layer. The
muscularis is responsible for moving urine through the ureters and into the
bladder.
- The outer layer of adventitia.
The urinary bladder:
Thetrigone.
- The internal urethral sphincter At the bladder-urethra junction,
involuntarily sphincter closes off the urethra and prevents the leakage of
urine when you are not voiding.
- The external urethral sphincter at the distal end of the urethra at the
orifice closes off the urethra and prevents the leakage of urine when you
are not voiding.
The urethra is a small muscular tube that transports urine
from the bladder out of the body. The urethra is five times longer in males (8
inches, 20 cm) than in females (1.6 inches, 4 cm). The course of the urethra
also differs between the sexes. At the bladder-urethra junction, the internal
urethral sphincter involuntarily sphincter closes off the urethra and prevents
the leakage of urine when you are not voiding.
Regulation of Glomerular Filtration
In a healthy body, GFR remains relatively constant even in the face of
substantial changes in arterial blood pressure. By adjusting resistance to the
flow of blood, renal autoregulation prevents significant
fluctuations in GFR when systemic arterial blood pressure rises or falls. Two
mechanisms are involved in this intrinsic control. The myogenic mechanism
results from the inherent tendency of vascular smooth muscle to contract
when stretched. This means that the diameter of afferent arterioles changes in
response to fluctuations in blood pressure. Increasing blood pressure in the
afferent arteriole stretches the smooth muscle in the wall of the arteriole. As
a result, the smooth muscle will contract and the arteriole will vasoconstrict.
This reduces the diameter of afferent arterioles, and blood flow. Decreasing
blood pressure in the afferent arteriole removes the stretch of the smooth
muscle in the wall of the arteriole. As a result, the smooth muscle will relax
and the arteriole will vasodilate. This increases the diameter of afferent
arterioles and blood flow. In both cases, the result is a relatively stable
GFR.
The second element of renal autoregulation is the tubuloglomerular feedback
mechanism The macular densa cells of the distal convoluted tubule are
part of the juxtaglomerular apparatus and are responsible for the
tubuloglomerular feedback mechanism. The macula densa cells respond to the
sodium concentration in the filtrate that flows from the ascending limb of
nephron loop into the distal convoluted tubule. The sodium concentration in the
filtrate is directly related to the rateglomerular filtration rate (GFR). When
GFR is high, there is not enough time for reabsorption, and the filtrate will
have a high sodium concentration. The macular densa cells respond to this high
sodium concentration by releasing the vasoconstrictor adenosine, which narrows
the diameter of the afferent arterioles. Reduced blood flow lowers the net
filtration pressure and the GFR, which enhances sodium chloride reabsorption.
Conversely, when GFR is low the filtrate will have a low sodium concentration.
The release of the paracrine agent by macula densa cells is inhibited. As a
result, the afferent arterioles dilate and increase tje blood flow into the
glomerulus. The result is to increase net filtration pressure in the glomerulus
and increase GFR. This has the opposite effect of macula densa cell activation:
it increases the amount of filtered sodium, and it reduces sodium reabsorption.
These two autoregulatory mechanisms help keep the flow of blood through the
kidneys relatively constant when mean systemic arterial blood pressure is within
a range of approximately 80 mm Hg to 180 mm Hg. However, autoregulation cannot
adjust for changes in systemic blood pressure that are outside of this
range.
Neural Regulation
The sympathetic nerve fibers that innervate renal blood vessels provide
an extrinsic regulatory mechanism for GFR. During extreme stress or
blood loss, the sympathetic nervous system must meet the needs of the
body as a whole, for example, by temporarily reducing kidney activity
and redirecting blood to other vital organs. In such situations, neural
controls override renal autoregulation. The sympathetic nerve fibers
release the neurotransmitter norepinephrine. Norepinephrine activates
alpha-adrenergic receptors on vascular smooth muscle
and causes afferent arterioles to constrict. The resulting reduced blood
flow into glomerular capillaries lowers net filtration pressure and GFR.
This decreased renal blood flow helps maintain blood volume by reducing
urine output and increasing perfusion to other body tissues.
Hormonal Regulation
Hormones also regulate GFR. One such mechanism is activated when
jutaglomerular (JG) (or granular) cells are stimulated to secrete the
enzyme renin. This enzyme begins a process that results in the
production of angiotensin II, a powerful systemic vasoconstrictor that
also constricts the afferent and efferent arterioles. Angiotensin II
also stimulated the contraction of mesangial cells resulting in a
decrease in the surface area of the glomerulus. Because renin is
released when blood pressure is low, the resulting constriction of the
efferent arteriole helps maintain net filtration pressure in the
glomerular capillary and consequently, GFR. Another hormone, atrial
natriuretic peptide (ANP) increases GFR. ANP is released when the atria
of the heart is stretched, for example, in heart failure when blood
volume increases due to sodium and water retention. ANP relaxes the
mesangial cells of the glomerulus, making more surface area available
for filtration. This increases GFR.
Glomerular Filtration Regulation
| Regulation |
Primary Stimulus |
Mechanism/Activity Site |
Effect |
| Renal autoregulation |
Systemic rising or falling of arterial blood pressure |
Adjusts resistance to the flow of blood, when systemic arterial blood
pressure rises or falls |
Prevents significant fluctuations in GFR |
| Myogenic mechanism |
Smooth muscle fibers in walls of afferent arteriole are stretched when
blood pressure increases |
Contraction of smooth muscle fibers narrows lumen of afferent
arterioles |
GFR decrease |
| Tubuloglomerular feedback |
Increased delivery of sodium ions and chloride ions to the macula densa
when blood pressure increases |
Constriction of afferent arterioles due to the release of adenosine by
macula densa cells |
GFR decrease |
| Vasoconstrictor adenosine |
Decreased delivery of sodium ions and to the macula densa when blood
pressure drops |
Dilation of afferent arterioles due to inhibition of adenosine release
by macula densa cells |
GFR increase |
| Neural regulation |
Release of norepinephrine due to increased activity of renal sympathetic
nerves |
Constriction of afferent arterioles due to activation of
alpha-adrenergic receptors and renin release |
GFR decrease |
| Hormone regulation |
Stimuli cause justaglomerular cells to secrete renin. |
Once justaglomerular cells secrete renin, angiotensin II is
produced. |
Maintains GFR |
| Angiotensin II |
Production of angiotensin II due to decreased blood volume or blood
pressure |
Constriction of afferent arteriole and contraction of mesangial cells |
GFR decrease |
| ANP |
Secretion of ANP due to stretching of atria of heart |
Capillary surface area available for filtration increased due to
relaxation of mesangial cells in glomerulus |
GFR increase |
Hormonal Regulation of Reabsorption and Secretion
Five hormones control the absorption of water, and sodium, chloride, and calcium
ions: angiotensin II, aldosterone, antidiuretic hormone, atrial natriuretic
peptide, and parathyroid hormone.
Let’s look at each:
Angiotensin II
The renin-angiotensin-aldosterone system is stimulated when blood volume
and blood pressure decrease. A decrease in blood pressure reduces the
amount of stretch in afferent arteriole walls and stimulates
juxtaglomerular cells to release renin into the blood. Renin release is
also stimulated directly by sympathetic nerve fiber activity. Renin sets
in motion a cascade of events that leads to the production of the
hormone angiotensin II.
Angiotensin II plays three primary roles. First, it constricts afferent
arterioles, resulting in a reduction in GFR. Second, it stimulates an
exchange mechanism. This leads to an increase in the reabsorption of
water, sodium ions, and chloride ions. Finally, the hormone prompts the
adrenal cortex to secrete the hormone aldosterone.
Aldosterone
The release of aldosterone from the adrenal cortex is stimulated by the
presence of angiotensin II as well as an increased concentration of
potassium ions. Aldosterone stimulates the insertion of sodium channels
in the apical membrane and sodium/potassium pumps in the basolateral
membranes of the principal cells of the distal convoluted tubules and
the collecting ducts. The result is an increase in the reabsorption of
sodium ions. Aldosterone also increases the secretion of potassium ions
by principal cells in the collecting duct. Because of the increased
reabsorption of sodium ions, more water is reabsorbed and blood volume
is effectively increased.
Antidiuretic hormone (ADH)
Antidiuretic hormone (ADH)acts to decrease urine production by increasing
the permeability of principal cells in the late distal convoluted tubule
and the collecting duct, thus increasing facultative water reabsorption.
Without ADH, the apical membranes of principal cells are relatively
water-impermeable. ADH also stimulates the insertion of aquaporins in
the apical membrane, allowing water molecules to move more quickly from
tubular fluid into the cells and then through the always fairly
permeable basolateral membrane and into the blood. When you are
dehydrated, ADH is released, and the kidneys conserve
water by producing a small volume of very concentrated urine. ADH
secretion is controlled bv a negative feedback system. When
the water concentration of plasma and interstitial fluid decreases
(i.e., when osmolarity increases), more ADH is secreted into the blood,
making principal cells more water-permeable. This restores plasma
osmolarity towards normal.
Atrial natriuretic peptide
Atrial natriuretic peptide is released from the heart in response to
large increases in blood volume. This hormone inhibits the reabsorption
of water and sodium ions in the proximal convoluted tubule and
collecting duct. It also inhibits aldosterone and ADH secretion. The
resulting increased sodium ion excretion in urine and the increased
urine output lower blood volume and pressure.
Parathyroid hormone (PTH)
Parathyroid hormone (PTH) affects calcium and phosphate ion reabsorption.
It is released by the parathyroid glands in response to a reduced
concentration of calcium ions in the blood. PTH stimulates increased
reabsorption of calcium ions in the early distal convoluted tubule. PTH
also inhibits the reabsorption of phosphate ions in the proximal
convoluted tubule, prompting the excretion of phosphate ions in the
urine.
Hormones Involved in the Regulation of Reabsorption and
Secretion
| Hormone |
Stimulus for Release |
Result |
| Aldosterone |
Increased levels of angiotensin II and plasma potassium ion (K+) |
Increased secretion of K+ and reabsorption of sodium ions (Na+) and
chloride ions; increased water reabsorption, leading to increased blood
volume |
| Angiotensin II |
Reduced blood volume or reduced blood pressure |
Increased reabsorption of solutes (including Na+) and water, leading to
increased blood volume |
| Antidiuretic hormone |
Increased osmolarity of extracellular fluid or decreased blood
volume |
Increased facultative reabsorption of water, leading to reduced
osmolarity of body fluids |
| Atrial natriuretic peptide |
Stretching of atria of heart |
Increased excretion of Na+ in urine; increased urine output reduces
blood volume |
| Parathyroid hormone |
Decreased plasma calcium ion (Ca2+) level |
Increased Ca2+ reabsorption, leading to decreased reabsorption of
phosphate ions in the proximal convoluted tubule and increased excretion
of phosphate ions in urine |
Homeostasis Functions of the Kidneys
The kidneys do most of the work of the urinary system. In addition to being the
major excretory organs, the kidneys are important regulators of the volume and
chemical composition of blood, and they maintain the correct balance between
water and salts, and between acids and bases, in the body. They also regulate
blood glucose levels, produce hormones, and metabolize vitamin D to its active
form, calcitriol.
| Function |
Description |
| Regulate ionic composition of blood |
Homeostatic maintenance of plasma levels of ions, including sodium,
potassium, phosphate, calcium, and chloride ions |
| Regulate blood pH |
Buffer hydrogen ion levels in the blood by excreting some in urine and
by conserving bicarbonate ions, which buffer hydrogen ions in the
blood |
| Regulate blood volume |
Either conserve water or eliminate it in urine |
| Regulate blood pressure |
Secrete renin, which activates the renin-angiotensin-aldosterone system
and increases blood pressure |
| Maintain blood osmolarity |
Regulate water loss and solute loss in urine |
| Produce hormones |
Help control calcium homeostasis with calcitriol and stimulates the
formation of red blood cells with erythropoietin |
| Regulate blood glucose |
Perform gluconeogenesis, releasing glucose into blood to maintain normal
levels |
| Excrete waste/foreign substance |
Form urine, which eliminates unneeded substances, wastes from metabolic
reactions (e.g., ammonia, bilirubin, uric acid), and foreign substances
(e.g., drugs, environmental toxins) |
Aging and Urinary System Homeostasis
Aging affects all body systems, but perhaps none undergoes as many age-related
changes as the urinary system. Among the physical changes in urinary tract
function that occur with aging are decreases in bladder capacity and bladder
emptying, loss of sphincter muscle tone, and a reduced ability to delay voiding.
In addition, age-related conditions such as stroke and Alzheimer's disease can
affect the micturition center in the brain. These are some of the reasons why
urinary incontinence is so common in the elderly, affecting up to a third of
older men and more than half of older women. Age-related changes in the kidneys
include a decrease in organ size, decrease in renal blood flow, and impaired
sodium conservation. The number of functional nephrons and the glomerular
filtration rate (GFR) also decline with age. In fact, only about two percent of
adults over age 70 have normal renal function. In about two thirds of the
elderly, the GFR rate is less than 60 milliliters (2 ounces) per minute compared
with the normal rate of 120 milliliters (4 ounces) per minute. This has
important implications for the many drugs used to treat a variety of age-related
conditions. Medication dosages must often be adjusted to compensate for the
reduced renal clearance.
Common Dysfunction of the Urinary System
As noted earlier in the unit, the entire process of urination is known as
micturition. A healthy, well functioning urinary system begins with urine being
produced by the nephrons in the kidneys, continues via the process of
peristalsis bringing the urine into the bladder, and ends with urine exiting
through the urethra. However, the urinary system sometimes is compromised when
it is permeated by bacteria. Thus far, we have discussed two dysfunctions: renal
ptosis, where the fat deposit that holds the kidneys in place fails resulting in
one or both kidneys dropping into the pelvis, and urinary tract infections, the
most common type of urinary bacterial infections. Later on in the unit, you be
introduced to kidney stones, deposits that occur within the urinary tract.
Several other urinary disorders are also discussed.
Pyelonephritis
A specific type of kidney infection, pyelonephritis, starts in either the
bladder or urethra and ultimately migrate to the kidneys. If the
infection does not move to the bladder, it is then referred to as
cystitis. Common causes of Pyelonephritis and cystitis are either
Escherichia coli or sexual activity and may include flu like symptoms
such as fever, vomiting, chills, nausea, and/or frequent, painful,
urination. Pyelonephritis and cystitis are usually treated with
antibiotics.
Kidney Stones
Kidney stones or renal caculi are relatively large calcium deposits that
occur within the urinary tract. Much like pyelonephritis, symptoms can
be flu like and include vomiting, nausea, fever, chills. However, the
flu like symptoms are usually in combination with flank pain, or pain
located one side of the body at the upper abdomen and lower back. Common
causes of kidney stones include obesity, dehydration, and a calcium rich
diet. Treatment can range from medicinal to lithotripsy, a surgery that
employs shock waves to break up the stones to a size that can be passed
through the urinary tract, to uteroscopy, a surgery that inserts a scope
through the urethra, urinary bladder, and ureter to beak and remove the
stone.
Kidney Failure
Kidney failure, also called renal insufficiency, is a medical condition
wherein the kidneys cannot filter enough toxins and urea from the blood
to maintain proper homeostasis. Kidney failure can
occur as either acute and chronic problems. Many of the symptoms of
kidney failure as associated with the build up of toxic elements in the
blood including:
- increased urea
- increased phosphates
- increased potassium
- general fluid retention
Those in the beginning stages of renal insufficiency might experience
swelling from the body retaining fluids. In addition, initial symptoms
might include bloody stools, a metallic taste in the mouth, and easy
bruising. Treatment for renal insufficiency varies depending on the
stage and can include: dietary changes (diet high in carbohydrates
and low in protein, salt, and potassium), antibiotics, diuretics
to help remove fluid, and/or renal replacement therapy. Renal
replacement therapy involves dialysis treatment to aid in the removal of
the toxic elements from the blood.
Let’s summarize
Regulation of glomerular filtration
Glomerular filtration (GFR) remains relatively constant due to renal
autoregulation. Renal autoregulation is comprised of:
- The myogenic mechanism results from the vascular smooth muscle
contracting when stretched.
- The tubuloglomerular feedback mechanism that results in the macular
densa cells responding to the filtrate’s high sodium concentration
by releasing paracrine agent, that causes vasodialation diameter of
the afferent arterioles and reduces blood flow.
GFR is regulated by:
-
Sympathetic nerve fibers: During extreme
stress or blood loss, the sympathetic system will meet the needs of
the whole body. The sympathetic nervous can override renal
autoregulation and temporarily reduce kidney activity
-
Hormones: The enzyme renin produces
angiotensin II. When blood pressure is low, Angiotensin II helps
maintain pressure in the glomerular capillary and consequently, GFR
-
Atrial natriuretic peptide (ANP) increases GFR
by relaxing the mesangial cells of the glomerulus, making more
surface area available for filtration. This increases GFR.
Hormonal Regulation of Reabsorption and Secretion.
Five hormones control the absorption of water, sodium ions, chloride
ions, and calcium ions:
-
Angiotensin II: Angiotensin II stimulates the
adrenal cortex to secrete aldosterone.
-
Aldosterone: Aldosterone stimulates an
increase in secretion of potassium and reabsorption of sodium ions
and results in increased water reabsorption, leading to increased
blood volume.
-
Antidiuretic hormone (ADH): ADH decrease urine
production by increasing the water permeability of principal cells
in the late distal convoluted tubule and the collecting duct. This,
increases facultative water reabsorption.
-
Atrial natriuretic peptide: This is released
from the heart in response to large increases in blood volume. The
action of ANP inhibit reabsorption of water and sodium ions in the
proximal convoluted tubule and collecting duct.
-
Parathyroid hormone (PTH): This is released by
the parathyroid glands in response to a reduced concentration of
calcium ions in the blood. It results in decrease in phosphate
reabsorption in the proximal convoluted tubule and an increase in
calcium reabsorption in the distal convoluted tubule.
Aging and urinary system homeostasis
When taking all body systems into account, none undergoes as many
age-related changes as the urinary system:
- decreased bladder capacity and bladder emptying,
- loss of sphincter muscle tone,
- reduced ability to delay voiding.
Age-related conditions such as stroke and Alzheimer's disease can affect
the micturition center in the brain.
Integration of Systems
The urinary system is responsible for only a part of our body’s ability to
exchange material with the environment. It is not the only excretory system in
the body. Several other organs, tissues, and processes are also involved in
excretion. These structures temporarily store wastes, transport them for
removal, or excrete the wastes and excess materials from the body.
Excretory Organs
| Organ System |
Organ |
Major Excretory Function |
| Digestive |
Large intestine |
Defecation removes solid waste and some water |
| Integumentary |
Skin/sweat glands |
Remove water, salts, other wastes |
| Respiratory |
Lungs |
Remove carbon dioxide |
| Urinary |
Kidneys |
Remove wastes and excess substances from blood |
The digestive system participates in waste removal in two ways. First, the liver
detoxifies some substances. One example is the conversion of ammonia into urea.
The large intestine is also considered an excretory organ. It eliminates a
number of substances through defecation, including undigested food, water,
carbon dioxide, water, salts, cholesterol and heat. The respiratory system may
seem like an unlikely waste remover, but when we exhale, the lungs excrete
carbon dioxide and also rid the body of heat and some water vapor. Finally, the
sweat glands in the skin are important excretory structures. They help dispose
of excess water, heat, and carbon dioxide, as well as small amounts of salts and
urea.
Integrations of Body Systems
The kidneys are critical organs because they regulate blood volume and blood
pressure. These organs perform this regulatory role by altering the amount of
water that is eliminated in urine, as well as by releasing the chemicals renin
and erythropoietin. When blood pressure drops, the kidneys secrete
renin, which is both a hormone and an enzyme. Renin activates a
hormonal pathway that increases blood pressure. When oxygen levels in body
tissues decrease, the kidneys secrete the hormone erythropoietin.
Erythropoietin stimulates the bone marrow to increase production of red blood
cells.
The kidneys regulate ion concentrations in blood plasma by returning some ions to the
bloodstream and by excreting excess ions in urine. They control blood pH by
adjusting the amount of hydrogen ions and bicarbonate ions that are reabsorbed or secreted in
urine. The kidneys also reclaim valuable nutrients from the filtrate and return
them to the systemic circulation.
The urinary system also affects all other body systems, whether directly or
indirectly.
Interactions Between the Urinary System and Other Body
Systems
| Body System |
Urinary System Interaction |
Effects |
| All Systems |
Eliminates metabolic wastes· Maintains fluid, electrolyte, and acid-base
balances |
Controls volume, pH, and composition of body fluids· Homeostasis |
| Cardiovascular |
Kidneys increase/decrease reabsorption of water filtered from blood·
Cells in kidneys release renin when blood pressure decreases |
Helps regulate blood volume and blood pressure·
Renin-angiotensin-aldosterone systemincreases blood pressure |
| Digestive |
Kidneys help synthesize calcitriol, the active
form of vitamin D synthesized in the liver |
Calcitrol is necessary for the absorption of dietary calcium |
| Endocrine |
Kidneys help synthesize calcitriol· Kidneys release erythropoietin |
Calcitriol is an endocrine hormone that promotes bone reabsorption·
Erythropoietin stimulates red blood cell production |
| Integumentary (skin) |
Kidneys work with skin to synthesize calcitriol· Kidneys maintain fluid
balance |
Calcitriol is necessary for the absorption of dietary calcium· Fluid
balance necessary for perspiration |
| Lymphatic/ immune |
Kidneys increase/decrease reabsorption of water filtered from blood |
Helps regulate volume of lymph and interstitial fluid |
| Muscular |
Kidneys help regulate calcium and phosphate levels in blood |
Calcium and phosphate balance important for muscular contractions |
| Nervous |
Kidneys are responsible for gluconeogenesis
|
Glucose is necessary for the production of adenosine triphosphate in
neurons |
| Reproductive |
Urethra is dual passageway in males· In pregnant women, urinary system
eliminates wastes for mother and fetus |
Allows passage and direction of sperm· Eliminates metabolic wastes of
fetus |
| Respiratory |
Kidneys work with lungs to adjust pH of body fluids |
Prevent abnormal function of enzymatic pathways |
| Skeletal |
Kidneys help regulate calcium and phosphate levels in blood |
Calcium and phosphates are essential for bone deposition |
Let’s summarize
The urinary system is one of several excretory systems in the body:
- The digestive system participates in waste removal in
two ways.
- Liver detoxifies some substances.
- Large intestine eliminates substances through defecation (undigested
food, water, carbon dioxide, water, salts, cholesterol and heat).
- The respiratory system removes waste through
exhalation (carbon dioxide, some heat and some water vapor).
- The sweat glands help dispose of excess water, heat,
and carbon dioxide.
The kidneys have an endocrine function.
-
Renin is an enzyme secreted by the kidneys that
initiates the pathway leading to the production of angiotensin II and the
secretion of aldosterone.
-
Erythropoietin is secreted by the kidneys when oxygen
levels in the kidney decrease.
For the cardiovascular system, the urinary system helps regulate blood volume and
blood pressure.
For the digestive system, the kidneys produce calcitrol, the functional form of
vitamin D necessary for the absorption of dietary calcium.
For the endocrine system, calcitriol promotes bone reabsorption.
For the integumentary system, the skin and kidneys both paly a role in the
production of calcitriol.
For the lymphatic system, the urinary system helps regulate the volume of lymph
and interstitial fluid.
For the muscular system, the urinary system helps with calcium and phosphate
balance for muscular contractions.
For the nervous system, the urinary system is responsible for some
gluconeogenesis to insure the availability of glucose for neuron metabolism.
For the reproductive system, the urinary system allows for the passage and
direction of sperm in males and eliminates metabolic wastes of the fetus (via
the maternal cardiovascular system)
For the respiratory system, the urinary system helps maintain the acid base
balance.
For the skeletal system, the urinary system assists with maintenance of calcium
and phosphate balance that is essential for bone deposition.
For all body systems, the urinary system helps with homeostasis by influencing
the volume, pH, and composition of body fluids.
Take any anatomy and/or physiology textbook or course, and chances are that the chapter
dedicated to the lymphatic system will be short and lacking detail when compared to its
mighty and well known sibling, the cardiovascular system. Maybe because of the
prominence and the romantic notions ascribed to the heart and blood, the cardiovascular
system has been studied much more extensively. Since its original description by
Hippocrates, the lymphatic system has been neglected by both scientific and medical
communities because of its vagueness in structure and function. “Rediscovered” in the
1600s as the venae albae et lacteae (“milky veins”), the lymphatic system was for long
considered a secondary vascular system that supports the blood vascular system.
In fact, the lymphatic system has important functions ranging from transport of fats,
returning leaked fluids to the blood, to housing the cells and many organs of the immune
response.
Follow this link to review the overview
of this unit presented in Unit 1: Introduction of Systems.
Let’s start our discussion by examining the Big Ideas of Anatomy and Physiology and exploring
some ways these Big Ideas may be seen in the functioning of the Lymphatic System and Immunity.
These ideas have been identified as common throughout the body systems, and will provide
secure footing as we begin to build our understanding of these key functions.
-
Living organisms are causal mechanisms whose functions are to be understood by
applications of the laws of physics and chemistry.
When molecules and atoms leave tissues and enter the lymph vessels as lymph fluid the
driving forces behind those movements are chemical processes, namely osmosis and
diffusion. Movement of the lymph fluid relies on capillary action as wells as differential
changes in the pressure on the fluid inside the lymph vessels. The immune function of the
lymphatic system uses the partial charges and shapes of larger biomolecules like proteins
to determine the fit of a cell surface receptor for a variety of molecules. In the immune
and lymphatic system recognition of histocompatibility markers by cell surface receptors
and recognition of foreign antigen by the immune cells relies on these underlying
properties of molecules
-
The cell is the basic unit of life.
A key piece of the lymphatic and immune systems is the bone marrow, which produces white
blood cells that can protect the body by attacking pathogens that have entered the blood
stream. The bone marrow contains stem cells which are not only capable of replication but
remain multipotent. The division of the stem cells and further differentiation of those
daughter cells into functioning cells of the immune system allows the body to defend
against foreign invaders as well as monitor cells which may have change and are growing in
way which could disrupt homeostasis.
-
Life requires information flow within and between cells and between the
environment and the organism.
The cornerstone of the immune system is its ability to distinguish self from non-self, in
the molecular sense. Once the recognition has occurred, multiple waves of information
within the cells and between cells take place, mobilizing immune cells and provoking
changes in them in order to attack and destroy the invader. In addition, damaged tissues
and cells release signalling molecules which activate not only other cells but also
protein cascades which promote blood clotting, wound healing and activation of a several
kinds of cells which may be involved in the immune response.
-
Living organisms must obtain matter and energy from the external world. This matter and
energy must be transformed and transferred in varied ways to build the
organism and to perform work.
Energy ingested and digested can be stored as high energy molecules like ATP but can also
be used to construct large biological molecules like those found in and on cell membranes,
as well as RNA and DNA. Cell functioning requires proper transcription and translation of
the DNA and well as accurate replication of the DNA when the cell divides--in other words,
the DNA runs the machinery of the cell. In the lymphatic system that energy is used for
the replication of cells to build and maintain the lymphatic vessels, the cells of the
immune system which fight pathogens directly and the cellular manufacture of antibodies
which are proteins and require energy for their manufacture. Additionally, some degree of
fever is beneficial for the immune response as it enhances metabolism, and that way
increases the activity of the cells.
-
Homeostasis (and “stability” in a more general sense) maintains the internal
environment in a more or less constant state compatible with life.
The lymphatic system contributes to homeostasis in several ways. The lymph and lymph
vessels drain away excess fluid and molecules which could contribute to edema and swelling
disrupting tissue integrity. The immune response helps to neutralize pathogens which may
not only disrupt homeostasis on a local tissue level but could result in a systemic
disruption as well. Likewise, loss of control of lymphatic functions which would normally
contribute to maintaining homeostasis can also lead themselves to a disruption of
homeostasis. Inflammation should stop as soon as it is not necessary anymore, because the
presence of those powerful molecules may cause damage to tissues. Many conditions are
caused by an inflammatory process that goes on, such as rheumatoid arthritis or Crohn’s
disease.
-
Understanding the behavior of the organism requires understanding the relationship
between structure and function (at each and every level of organization).
Lymphatic vessels have larger openings than blood vessels allowing them to pick up and
transport larger molecules back to blood circulation. B lymphocytes with a small cytoplasm
become, upon the right stimulus, plasma cells with large amounts of rough endoplasmic
reticulum churning out amazing amounts of antibodies. A set of small plasma proteins,
called complement, self-assemble to form a deadly ring that opens a hole in the membranes
of invading cells. Lymph nodes and and the spleen have much larger total cross-sectional
areas than the vessels feeding into them. This structural arrangement decreases the
velocity of flow through these structures, providing more time to detect and react with
foreign pathogens.
-
Living organisms carry out functions at many different levels of
organization simultaneously.
Generally speaking the molecules of the immune system, antibodies, complement, cell
surface receptors, cell recognition molecules and pathogen recognition molecules stimulate
a response within the appropriate cells of the immune system. The cellular immune
responses may result in changes to the affected tissues such those found associated with
the inflammatory response. The lymph fluids, lymph vessels and other specialized lymph
structures continue to support the immune response while also providing a means for the
tissues to remove excess fluids, cellular debris and dead and dying cells and pathogens.
-
All life exists within an ecosystem made up of the physiochemical and
biological worlds.
The body can be viewed as interacting with two ecosystems, 1) the external ecosystem of
the world around us and 2) the internal ecosystem, which contains symbiotic bacteria and
in which your cells and tissues must function. The lymphatic system serves at the
interface of both those ecosystems. The immune function modulates the impact that other
organisms living in the external ecosystem may have as pathogens. The lymphatic impact on
the internal ecosystem involves not only immune function but includes the inflammatory
response to tissue injury, the transport of large lipid molecules away from the digestive
system and helping to maintain fluid and molecular balances for a healthy internal
ecosystem.
-
Evolution provides a scientific explanation for the history of life on
Earth and the mechanisms by which changes to life have occurred.
The lymphatic and immune systems have become more complex during evolution. But even in
the context of the immune response we can observe examples of evolutionary changes. For
one, many microbes mutate (change) in a short time, provoking the immune system to develop
a new response each time (ever wondered why you need a new flu shot every year?) On the
other hand, the antibodies produced during the immune response can also be switched to be
complementary to new antigens, resulting in more effective antibodies.
Humans live with organisms inside their internal environment as well as free-living
organisms found in the external environment. Random mutation in genetic material is the
source for change in an organism or species. Must mutations are either neutral or
deleterious. A very small percentage of mutations result in new trait or character which
allows that organism to thrive in a changing environment. Organisms which have rapid
reproductive cycles can change much more quickly as a species than organisms which have a
long reproductive cycle. In bacteria the reproductive cycle is often hours compared to
humans which may be 20+ years. The lymphatic system can adapt to changes in pathogenic
organisms by rearranging the genes used to make antibodies, activating a different clone
of lymphocytes or altering the expression of cell surface molecules on white blood cells.
Click on the different pieces of the lymphatic system, identified in the image at right,
to learn about how the Big Ideas are exemplified in the functioning of the lymphatic and
immune systems and show that you understand these connections by completing the
activities below.
The Lymphatic System
Introduction
Our body is in constant exchange with the environment, through breathing,
eating and other activities. Therefore, it is important to screen the body
and its components regularly to identify foreign invaders that might enter
during these activities (or in any other manner). Further, it is important
to rapidly and effectively remove these invaders before they can cause
significant harm. Our body has specialized transport systems to carry out
these functions. The cardiovascular and lymphatic systems work together to
transport excess fluids (blood and lymph fluid, respectively) away from body
tissues. The cardiovascular and lymphatic systems also participate in the
function of immunity, helping defend the body's cells from foreign organisms
that may enter the body tissues or fluids.
The adenoids are an aggregation of lymphoid cells located in the posterior
superior wall of the nasopharynx.
Since the the adenoids are contained by a supporting network of reticular
fibers we refer to them as belonging to the category of lymphoid nodules.
They are positioned such that most of the air which is inhaled through the
nose passes across the surface of the adenoids. The adenoids structure and
function relates to several of the underlying foundations of physiology
known as the Big Ideas. The adenoids provide the first interface between the
external ecosystem and the internal one. When a pathogen or potentially
harmful foreign molecule crosses the surface of the adenoids, the cells in
the germinal center of the adenoids may be stimulated to divide to react the
harmful pathogen or molecule. The energy to do this has been stored within
the individual cells but ultimately came from the energy ingested and
processed through digestion.
The tonsils include both the palatine tonsils located posteriorly in the oral
cavity near when the pharynx the meets the soft palate and the lingual
tonsils, which are not visible because they are found near the base of the
tongue.
The structure and the function of the tonsils enable them to efficiently trap
particulate matter which may include bacterial, virus particles,mold spores,
and inert particles which may be either inhaled or ingested. Their location
near the junction of the oral cavity and the pharynx provides access to the
inhaled air of the respiratory system as well as ingested items. The
surfaces of the tonsils are highly convoluted increasing the surface area of
the interface with the external ecosystem. The highly convoluted nature of
the tonsils surfaces can work against this adaptation of this system. It is
possible for particles to become physically trapped in the folds of the
tonsils. In this situation the tonsils themselves may become infected and
abscessed and actually contribute to a disruption of homeostasis.
The thymus is "A lobular (of or pertaining to a lobe) structure, which
contains many immature, inactive lymphocytes. As the lymphocytes mature,
they leave the thymus to attack infected cells in lymphatic tissues
throughout the body." Follow this link to review the overview of this unit presented in Unit
1: Introduction of Systems.
The lobes of the thymus can be divided into an outer cortex region and an
inner medulla. The cortex region contains lymphoid stem cells. The daughter
cells of the lymphoid stem cells will differentiate into mature T cells. The
mature T cells migrate into the medulla and from their will enter the
bloodstream. T cells while being housed in the thymus are not active as
functioning T cells and are actually sequestered from the the antigens
circulating in the bloodstream. While maturing in the thymus the cells which
may be self- reactive are eliminated. The clones which survive and multiply
are those which do not react with self tissues. The thymus reaches its
greatest size during adolescence and then is gradually replaced with adipose
tissue.
The spleen is the largest of the lymphatic organs, and it houses lymphocytes
for potential immune response. The resident phagocytes within the spleen
perform the basic function of removing cell debris from the blood.
The spleen’s dark red color arises because it serves as a reservoir for a
large volume of blood. Consequently the blood flow through the spleen is
rather slow allowing it to remove abnormal blood cells, both red and white,
and other blood components through a process called phagocytosis. The spleen
is organized into red pulp and white pulp. The white pulp inside the splenic
nodule contains lymphocytes which may become active during an immune
response to foreign invaders.
Bone
marrow can be found in the cavities both compact and spongy bone. It is
frequently identified as either red marrow or yellow marrow. Red marrow
contains the pluripotent stem cells that divide and eventually produce the
myeloid stem cells which lead to the differentiation of erythrocytes,
granulocytes and monocytes. The pluripotent stem cells in the red marrow are
also capable of cell division and differentiation into the lymphoid line
which is responsible for T and B lymphocytes. Yellow bone marrow is
associated with the development of fat tissue. In times of extreme
physiologic stress such as extreme blood loss, yellow marrow is capable of
differentiating into red marrow which would increase the rate of blood cell
production.
Lymph
vessels are sometimes referred to as lymphatics. They carry lymph (tissue
fluid that has entered lymphy vessels) back to the venous blood system. Like
blood vessels the smallest lymphatics are referred to as capillaries. The
are very thin consisting of only endothelial cells. Unlike blood capillaries
lymph capillaries start as a blind pouch. There are gaps between the
endothelial cells so large that bigger molecules, like proteins and viruses,
have a way to get inside, since they would be too large to cross the
endothelial cell membrane. Similarly the red blood cells and white blood
cells can squeeze through the gaps to get inside. The capillaries flow into
lymphatic vessels. The lymph vessels are not connected to any kind of pump
and when standing they must work against the flow of gravity. To accomplish
this some of of the lymph vessels have one way valves to prevent backflow of
fluid. The larger lymph vessels actually have smooth muscle in their walls
which contracts rhythmically to help propel the lymph fluid in the lymph
vessels toward their connection to the venous blood system where it can be
recycled.
Lymph
nodes are small bean-shaped structures found along the lymph vessels. The
contain lymphocytes capable of clonally expanding with the correct antigen
stimulus and are designed to filter the lymph running through them. Lymph
nodes have and external capsule keeping the cells and the lymph separate
from other parts of the body. In addition, the velocity of flow is decreased
because the larger size of the lymph nodes increases total cross-sectional
area.
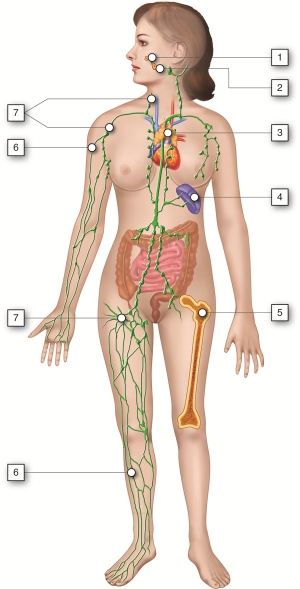
Follow this link to review the overview
of this unit presented in Unit 1: Introduction of Systems.
The lymphatic system is essential for our survival. This system has three main
functions:
- to collect and recycle the excess interstitial fluid and its dissolved
substances,
- to absorb fats and other substances from the digestive tract (this topic will be
discussed in the Digestive System Unit), and
- to initiate and coordinate an immune response to remove cellular debris, bacteria,
toxins, fungi, parasites, and viruses that accumulate in our bodies.
Because this system has the two very different functions of maintaining the proper fluid
balance in the body and protecting the body from harmful infections, we will begin its
study by 1) investigating the lympathic vessels and lymph which function in fluid
balance and then 2) investigate how these structures along with lymphatic cells, tissues
and organs function in protecting the body from infections. Infection can
be viewed as the invasion and multiplication of microorganisms that are not normally
present within the body. An infection may remain at the location where it entered the
body, or it may spread through the body via blood or lymphatic vessels.
Immunity is the state of having sufficient defenses (resistance)
against infections that might disrupt homeostasis. Immunity involves both non-specific,
inherent components (innate immunity) and specific, acquired from previous
exposure components (adaptive immunity).
It is important to realize that although immunity will be considered here in the context
of human anatomy and physiology, it is not restricted to humans or animals. The ability
to defend itself from “non-self” invaders appears as early as in bacteria defending
themselves from viral attacks, and it is an inherent homeostatic mechanism present in
all types of cells, plants, and animals. As organisms evolved, so did the immune system.
Thus, while the innate system is present in all animals, only vertebrates present the
adaptive response.
Lymph Tissue and Lymphatic Vessels
Like the circulatory system that carries blood throughout the body, the lymphatic
system is made of a series of vessels, capillaries, and organs. These structures
collect excess fluid and cellular debris from the tissues and return them back
to the blood.
In the circulatory system, blood flows from arteries, through capillaries and
into veins to be returned to the heart. On its way through the capillaries, some
of the fluid passes out across the capillary wall and into the interstitial
fluid in a process called capillary filtration. This filtration tends to occur
across the arterial end of the capillary, with most of the filtered fluid being
reabsorbed at the venous end of the capillary. This leaves a small amount of
fluid that remains in the interstitial spaces between cells. This filtered fluid
is mostly plasma plus any plasma proteins that might have leaked from the blood
vessel as well. This excess interstitial fluid is collected by the lymphatic
system. The fluid flows through the lymphatic vessels until it is returned to
the circulatory system to again become a component of blood. Once interstitial
fluid passes into lymphatic vessels, it is called lymph. Lymph is a clear,
pale-yellow fluid connective tissue.
The lymphatic system consists of many different tissues and organs that are found
throughout the body. Some organs provide the environment for the development and
maturation of leukocytes. Other tissues and organs trap pathogen and are the
sites where leukocytes can interact with the pathogen. The circulatory and
lymphatic systems interact to connect these organs and tissues.
 Vessels and Organs of the Lymphatic System
Vessels and Organs of the Lymphatic System
Fluid moves from blood capillaries into the interstitial spaces. Most of the
fluid returns to the blood, but some of the fluid moves from the interstitial
spaces into lymphatic capillaries to become lymph. To collect the lymph from the
interstitial space, lymph capillaries originate in the blood capillary beds, and
lymph vessels run parallel to the veins. At intervals along the lymphatic
vessels, lymph flows through lymph nodes. Fluid collected in the lymph system is
returned to the heart via veins in the chest. Unlike the circulatory system, the
lymphatic system does not flow through a closed, circular system. There are no
lymph arteries. Lymph fluid is not pumped around the body. Instead, the lymph
system collects the lymph into vein-like structures called lymph vessels and
returns it to the bloodstream.
Lymph Vessels and Capillaries
The lymphatic system contains both capillaries and vessels. Lymphatic vessels
begin as capillaries. Both of these structures are thin walled, which allows
lymph to be transported across the membrane and collected in the vessels.
Lymphatic capillaries have greater permeability than blood capillaries and can
absorb large molecules such as proteins and lipids. The endothelial cells that
make up the wall of a lymphatic capillary lack a basement membrane, loosely
attach to each other and slightly overlap. Interstitial fluid enters the
lymphatic vessel when the pressure is greater in the interstitial fluid than in
lymph and nothing in the interstitial fluid is excluded from entering the
lymphatic capillaries. When pressure is greater inside the lymphatic capillary,
the endothelial cells prevent lymph from passing back into the interstitial
spaces by acting like a one-way swinging door. Lymphatic capillaries are found
wherever blood capillaries are located except in the central nervous system and
bone marrow.
 Interaction of Blood and Lymphatic Vessels
Interaction of Blood and Lymphatic Vessels
 Anatomy of a Lymphatic Vessel
Anatomy of a Lymphatic Vessel
Lymphatic capillaries unite to form larger lymphatic vessels. Structurally,
lymphatic vessels are similar to veins because they also have one way valves
that function like gates to ensure the lymph only flows in one direction. Like
veins, skeletal muscle contraction exerts pressure on the lymph vessels and
forces the lymph forward through them. Lymph vessels are like one-way roads,
with the lymph being collected at the capillary beds and travels through the
body into the thoracic cavity. Lymph is deposited in one of two large ducts in
the chest region: the right lymphatic duct and the thoracic duct. The lymph then
travels from these ducts into venous circulation via the subclavian and jugular
veins.
Unlike the cardiovascular circulation, the lymphatic circulation lacks a pump
like the heart. Lymphatic vessels are low pressure vessels similar to veins and
the same muscle pump and respiratory pump that promote venous return also
facilitate lymph flow. Therefore, even though there is some smooth muscle in
lymphatic vessels, movement of the body is important to lymph circulation.
Common Dysfunctions of the Lymphatic Circulation
Anything that would disrupt the flow of lymph could contribute to significant
swelling of tissues (edema). Lymphedema is a condition of localized
fluid retention and a tissue swelling caused by a compromised lymphatic system.
Lymphedema can be primarily caused genetically or secondarily due to injury or
obstruction of lymphatic vessels. It is most frequently seen after lymph node
dissection, surgery and/or radiation, in which lymphatic system damage is caused
during the treatment of cancer, usually breast cancer. Lymphedema may also be
associated with parasitic infections in which parasites obstruct lymph vessels.
Symptoms may include fatigue, a swollen limb or localized fluid accumulation in
other body areas, including the head and neck, discoloration of the skin
overlying the swollen tissue and eventually deformity (elephantiasis).
Historical Perspective and Overview of Immunity
In relative terms, the study of immunity is a new science that started with
Edward Jenner’s discovery in 1796, that individuals exposed to cowpox were often
resistant to human smallpox. He also observed that people who had recovered from
even a mild case of smallpox were seldom infected a second time. Jenner
experimented with placing weakened (attenuated) strains of disease-causing
agents into otherwise healthy individuals to provide protection from disease. He
called his procedure vaccination. Adding to Jenner's work, in the 1880s, Robert
Koch and Louis Pasteur showed that most infectious diseases were caused by
microorganisms. Today it is generally accepted that these disease-causing agents
(pathogens) may be microscopic like viruses, bacteria, protozoa and yeast or
larger like molds and helminths. Scientists discovered a substance in the serum
of vaccinated individuals, which they termed antibodies, that could bind to the
pathogen that was used in the vaccination. It was shown that antibodies could be generated against a variety of substances and the
term antigen was created to describe these substances. As
we will learn shortly, not all antigens stimulate the immune system to produce
antibodies so a more general use of the term antigen refers to any substance
capable of being recognized during the immune response.
 by Gaston Mélingue Edward
Jenner vaccinating James Phipps, a boy of eight, on May 14,
1796. Public domain.
by Gaston Mélingue Edward
Jenner vaccinating James Phipps, a boy of eight, on May 14,
1796. Public domain.
Basically the immune system, as part of the lymphatic system, can be viewed as
may subsystems constantly guarding its host against microbial invasion. These
systems may be viewed both as an armory (chemical substances), with it tools and
weapons, and as an army (cells) capable of using these tools and weapons in
defense of the host. Immunity (resistance) has an innate component
and an adaptive component. Both of these components depend
on the responses of white blood cells
(leukocytes).
Innate immunity is the natural resistance with which a person is born and is the
result of actions of both external and internal systems. First lines of defense
against infection include mechanical and chemical barriers, such as skin and
saliva, the effectiveness of which is enhanced by antimicrobial substances.
Microbes that succeed in passing the external barriers next encounter the second
line of defense, the internal systems. The internal system includes
antimicrobial substances and subsets of leukocytes called granulocytes and
macrophages. Granulocytes contain an arsenal of cytoplasmic granules that can be
released during an immune response. Several of these granulocytes and the
macrophages are phagocytic which means they are able to ingest and
destroy pathogens. They use pattern-recognition receptors (PRRs) to
recognize pathogens. These receptors recognize and bind to molecules found on a
wide variety of microbial cells and on damaged or infected host cells. Thus they
recognize in a broad and general way the presence of harmful microbes and can
quickly attack and usually prevent the spread of the microbes. In addition, the
innate immune system includes complement, a set of soluble
molecules that can bind to certain molecules common to microbial cells. This
binding can lead to the direct destruction of the microbe and can also trigger
increased activity of phagocytic cells against the microbe.
A bridge between the innate and the adaptive components is the inflammatory
response. Although many soluble factors, blood proteins and cells
participate in this response, the main purpose of all of the factors is to
enable phagocytic leukocytes and plasma components to leave the blood
circulation and enter into damaged and/or infected tissues. The phagocytes in
the tissue carry out an array of activities at the inflamed site, the main one
being to rid the area of microorganisms and damaged tissue and thus to set the
stage for healing. As will be described more completely in a later module, all
events between the initial damage and the final restoration of the tissue may be
considered parts of the inflammatory response.
Sometimes, however, the innate immune components cannot quickly eliminate the
infectious agents especially viral infections. In such instances, cells of the
innate system interact with T lymphocytes (T cells)
and B lymphocytes (B cells) to initiate adaptive
immune responses against the threatening pathogens. The lymphocytes of the
adaptive immune response have receptors that are generated by random
rearrangement of DNA segments. Such receptors are able to identify and bind a
far greater range of substances than can be detected by the PRRS of the innate
response. Lymphocytes can detect, with great specificity, threats and
proliferate rapidly to act against them in a targeted manner.
The interaction between the innate and adaptive immune responses begins when
macrophages and dendritic cells process pathogens and display them in a way that
leads to activation of a subset of T lymphocytes (helper T cells).
The activated T helper cells can then interact with a variety of other cells,
including another subset of T lymphocytes (cytotoxic T cells) and
the B lymphocytes. Some cytotoxic T cells become directly involved in attacks
against the infection, while the B lymphocytes produce antigen-specific
antibodies. The function of antibodies in the immune system is to recognize and
neutralize microbes. Once inititated by cells of the innate response, adaptive
responses lead to an expansion of the numbers of lymphocytes able to recognize
and bind the pathogen in question. In responding to the pathogen, the
lymphocytes not only act directly on the substance providing the threat, but may
also recruit cells, for example phagocytic cells, and molecules, for example
complement, from the innate system and together both the innate and the adaptive
immune responses focus their destructive capabilities on removing the threat.
Unlike the innate response that operates at a relatively constant level,
adaptive immune responses generate memory B and T lymphocytes that produce more
vigorous responses upon subsequent encounters with the same microbe.
One essential component of the immune response is that it must be able to
distinguish self, which belongs in the body, from nonself (foreign). Agents or
molecules classified as nonself may enter the body from the outside or represent
an unacceptable change within the body (for example, a virus infected self-cell
or a self-cell becoming cancerous). B lymphocyte receptors recognize foreign
molecules not associated with self-cells (for example bacterial cells or their
toxins). However, T lymphocyte receptors recognize foreign molecules only in association with self-cells (for example a
virus-infected cell). Therefore, this recognition involves two considerations:
self versus nonself and threat versus nonthreat. Immune cells distinguish self
from nonself through cell-surface receptors. All nucleated cells of the body
express major histocompatibility complex (MHC)
molecules. MHC molecules associated with foreign proteins allow T lymphocytes to
recognize self that is threatened and needs to be removed by immune
responses.
In summary, because of the wide variety of pathogens located within the body and
at its surfaces, host defense requires a wide variety of recognition and defense
mechanisms. Innate immunity serves the first line of defense, but is unable to
recognize certain pathogens and unable to provide improved defenses that
prevents re-infection. Adaptive immunity is based on lymphocytes with receptors
that can potentially recognize any foreign antigen. In addition to the adaptive
immune response that can eliminate a pathogen, memory lymphocytes are generated
that can produce a more rapid and effective response on re-infection.
Common Dysfunctions of the Immune Response
The immune system works remarkably well. Unfortunately, at times it breaks down
and fails to function properly. Autoimmune diseases, such as systemic lupus
erythematosus (SLE), celiac disease and diabetes mellitus type I, arise from an
inappropriate immune response against components normally present in the body.
Allergies arise from an exaggerated immune reaction to agents that are not
normally harmful and lead to release of chemicals such as histamine. Acquired
Immunodeficiency Syndrome (AIDS) is caused by the human immunodeficiency virus
(HIV). HIV infects a subset of T cells in the body, thus compromising the immune
system. Cancers that affect either T or B cells are collectively called
lymphomas. Some are aggressive and fast-growing lymphomas, while others are
non-aggressive and slow growing. In a later module we’ll take a closer look at
these immune problems of clinical significance.
Your exploration of immunity in the following modules will extend from the simplest to
the most complex. Although the levels of organization are introduced in modules, the
functional interconnections between the organizational levels will build throughout the
development of modules within this unit.
-
Molecular level of organization includes 4 general categories of molecules:
- Three main types of antimicrobial substances (interferon, complement,
iron-binding transferrins)
- Substances that contribute to aspects of inflammation (histamine, kinins,
prostaglandins, leukotrienes, and complement)
- Molecules present on pathogens and infected self-cells
(pathogen-associated molecular patterns, antigens and major
histocompatibility complex) that are recognized by receptors (pattern
recognition receptors, B-cell receptor, T-cell receptor) on immune
cells
- Small protein hormones, called cytokines that stimulate or inhibit normal
cell functions such differentiation and growth
-
Cell level – Neutrophils, Eosinophils, Basophils, Monocytes, Macrophages,
Dendritic cells, Natural killer cells and B and T Lymphocytes
-
Tissue level – lymph and lymph nodules such as mucosa-associated lymph tissue and
tonsils
-
Organ level – lymph capillaries and vessels, primary lymph organs such as bone and
thymus, secondary lymph organs such as lymph nodes, and spleen
-
System level - all components that function together to drain excess interstitial
fluid and carry out immune responses
Types of Antimicrobial Proteins
Blood and interstitial fluids contain three main types of antimicrobial proteins
that interfere with microbial growth: 1) interferons, 2) complement, and 3)
iron-binding proteins.
- Virus-infected cells produce proteins called interferons
(IFNs). IFNs diffuse through the interstitial fluid
to uninfected neighboring cells. These proteins then cause the uninfected
cells to synthesize antiviral proteins that interfere with viral
replication. Viruses can cause disease only if they can replicate within
body cells. Although IFNs do not prevent attachment and penetration of
viruses to host cells, they do stop replication. Some interferons also have
important immunoregulatory and anti-tumor effects. There are three
families of IFNs; α-, β- and δ-IFN.
-
The complement system consists of 30 proteins. These proteins are
produced by the liver and circulate in the plasma. When activated, the
complement proteins collectively can cause cytolysis (bursting) of
cells, promote phagocytosis and contribute to inflammation. Most
complement proteins are designated by an uppercase letter C and a
number; and a few complement proteins referred to as factors B, D and P
(properdin). C1-C9 complement proteins act in a cascade. Complement
activation may begin by three different pathways: classical pathway requires antibodies bound to antigen, alternate pathway involves factors B, D and P
interacting with microbial molecules, and the lectin
pathway requires lectin binding on the surface of the microbe.
In all three pathways, C3 is activated and splits into fragments C3b and
C3a. Although the process of phagocytosis is discussed in another
module, C3b enhances phagocytosis by binding to the microbial membrane
(opsonization): phagocytic cells have receptors for C3b
enhancing the interaction between microbe and phagocyte. C3b fragments
together with proteins C5b-C9 initiates a series of reactions that bring
about cytolysis through formation of a pore shaped membrane attack
complex (MAC) inserted into the microbial
membrane. C3a and C5a bind to mast cells and cause histamine release and
the resulting increased permeability of blood vessels. C5a also attracts
phagocytes to the site of inflammation.
 Complement
Complement
- Iron is an important micronutrient most bacteria need to survive. By
reducing the amount of iron available, iron-binding proteins called
transferrins can inhibit the growth of certain
bacteria.
Substances that Contribute to Inflammation
Although the process of inflammation will be detailed in another module, two
immediate changes occur in blood vessels in the region of tissue damage and/or
infection; vasodilation of arterioles and increased permeability of capillaries.
Local immune cells release chemical substances that increase the permeability of
the local capillaries. Plasma and leukocytes circulating in the blood can now
move through the capillary wall and into the interstitial space. Localized
swelling, redness, heat and pain are produced. Among the substances that
contribute to these cardinal signs of inflammation are: 1) histamine, 2) kinins,
3) prostaglandins (PGs), 4) leukotrienes (LTs) and 5) complement.
-
Histamines are released by mast cells found in connective
tissue, basophils found in tissues where allergic reactions are occurring
and platelets found in blood in response to tissue injury. Histamine
promotes vasodilation and increased permeability of capillaries.
-
Kinins form in the blood from inactive precursor proteins
called kininogens. In addition to promoting vasodilation and increased
permeability, kinins attract phagocytes into inflamed tissues. Bradykinin is
an example of a kinin. Kinins affect some nerve endings causing much of the
pain associated with inflammation.
-
Prostaglandins (PGs) are lipids released by
damaged cells that intensify the effects of histamine and kinins. PG may
also stimulate movement (chemotaxis) of phagocytes through the capillary
wall, regulate smooth muscle contraction and intensify and prolong the pain
associated with inflammation.
-
Leukotrienes (LTs) are produced by basophils and
mast cells and promote increased permeability of capillaries. They also
function in chemotaxis and pathogen adherence of phagocytes.
-
Complement components (C3b and C5a) stimulate histamine
release, function in chemotaxis and phagocytosis.
The immune system produces a powerful response to foreign molecules, but does not attack
its own molecules. To avoid attacking self, the body has established several mechanisms.
The immune response can differentiate between pathogenic organisms (bacteria, viruses,
parasites and fungus) and human cells.
Pathogen-associated Molecular Patterns (PAMPs) and Pattern Recognition Receptors
(PRR)
Pathogens express pathogen-associated molecular patterns (PAMPs)
that are common to groups of pathogens, but are generally not found on
human cells. Examples of PAMPs include parts of bacterial cell walls, such as
certain lipopolysaccharide (Gram-negative bacteria), techoic acid (Gram-positive
bacteria) and peptidoglycans (bacteria); flagellin in bacterial flagella; and
various viral RNA molecules. In the past years DAMPs (Damage-associated
molecular patterns) have been added to the list of molecules that can initiate
the innate response. DAMPs include nuclear and cytosolic components than can
provoke a non-infectious inflammatory response, for example after tissue injury
or in gout. Pattern recognition receptors (PRR) found on the
surface of macrophages, neutrophils and dendritic cells bind the PAMPs as part
of the innate immune response. Molecules with pattern recognition ability may
also be soluble, like lysozymes and the complement components.
 Pathogen Associated Molecular Patterns
Pathogen Associated Molecular Patterns
Antigens and Antigen Receptors
Antigens are substances that bind to receptors on immune cells.
Entire microbes or parts of microbes may act as antigens. The term
antigen is derived from its function as an antibody generator. Chemical components of bacterial structures
such as flagella, cell walls and bacterial toxins, viral capsules, pollen, and
incompatible blood cells and tissue transplants can all act as antigens.
Typically, just small parts of a large antigen molecule act as the triggers for
immune responses. These small parts are call epitopes or
antigenic determinants. Antigens are most often large, complex
molecules such as proteins, however, nucleic acids, lipoprotein, glycoprotein
and some polysaccharides may also act as antigens. Simple, repeating subunits,
like cellulose and most plastics are not usually antigenic. Antigenicity is the ability to combine specifically with the secreted
antibodies and/or surface receptors on T-cells.
 Antigen Recognition
Antigen Recognition
T and B lymphocytes, also called T and B cells, are the mediators of the adaptive
immune response. They are lymphocytes with receptors that bind to antigens.
Individual T and B cells recognize only one antigenic determinant. This is like
a lock and key system where only one key can open the lock. The antigenic
determinant is the key. The lock is the receptor on the T or B cell. This
T cell receptor (TCR) or B cell
receptor (BCR) only fits that one key and opens it
(activates it) to initiate an immune. Because T and B cells only recognize one
antigen, each T and B cell is said to be antigen-specific.
One feature of the human immune system is its ability to recognize and bind to a
least a billion different antigens. Before an antigen enters the body, a small
number of T and B cells that can recognize and respond to that intruder are
ready and waiting. The diversity of antigen receptors in both T and B cells
results from shuffling and rearranging gene segments. This process is called
genetic recombination. As the lymphocytes are developing in the
bone marrow or thymus, gene segments are put together in different combinations.
The situation is similar to having a deck of 52 cards and dealing out three
cards. If you did this over and over you could generate many more than 52 sets
of three cards. Because of genetic recombination, each T and B cell has now
created a unique gene that codes for its unique antigen receptor. Because this
random rearrangement of gene segments could produce a TCR or BCR against self
antigens, B and T cells must be selected for self-recognition and tolerance.
While developing in the bone marrow, B cells that have an antigen receptor that
recognizes self-antigens are deleted. T cells that have an antigen receptor that
recognizes self-antigens are deleted in the thymus. However, to function
properly T cells do not just recognize antigen alone. They must have the ability
to recognize antigen in association with their own MHC complex.
Major Histocompatibility Complex (MHC)
Located in the plasma membrane of all nucleated body cells are thousands of
transmembrane glycoproteins called major histocompatibility complex
(MHC) antigens. Because they were first identified on
leukocytes they are also called human leukocyte antigens
(HLA). Unless you have an identical twin your MHC antigens are
unique. MHC molecules mediate interactions of leukocytes with other leukocytes
or body cells. Their normal function is to mark your cells as “self” cells, as T
cells will recognize those MHC molecules that do not match your own as foreign.
Such recognition can be one of the first steps in any adaptive immune response.
Protein molecules are continually made and broken down in a cell. Each MHC
molecule combines with a small part of a protein present in the cell (epitope),
similar to a hot dog (epitope) in a bun (MHC). The MHC then displays this
complex on its membrane surface. The epitope that is presented can either be
self or nonself. MHC molecules will become transmembrane molecules only after
they associate with an epitope.
 MHC-I
MHC-I
 MHC-II
MHC-II
The membrane bound MHC fall into two subgroups. Class I MHC
(MHC-I) are inserted into the plasma membrane of all nucleated
cells of the body. Class II MHC (MHC-II) are only
present on antigen-presenting cells (APCs) such as macrophages and B cells.
Class I MHC molecules combine with endogenous (intracellular) self peptide
fragments in the rough endoplasmic reticulum and should not react with cytotoxic
T cells. If a nonself peptide is present inside the cytosol of the cell, such as
during viral infection or cancerous transformation, the foreign epitope will
associate with Class I MHC. In this situation cytotoxic T cells may now
recognize that this cell is a threat and needs to be destroyed. Class II MHC
molecules combine with exogenous (extracellular) peptide fragments that have
been phagocytosed or endocytosed in vesicles. Lysosomes fuse with these vesicles
and cleave the uptaken protein into smaller peptides. These epitopes now are
loaded in the class II MHC molecules. Helper T cells with the appropriate
receptors bind with class II MHC/epitope molecules. During development, T cells
that recognize self-protein epitopes associated with MHC are eliminated during a
process called clonal deletion. Therefore, only MHC molecules complexed with a
foreign peptide will bind to T cell receptors (TCRs).
 Role of Major Histocompatibility Complex
Role of Major Histocompatibility Complex
Antibodies
Unlike T cells, B cells do not recognize antigen associated with MHC. Their B
cell receptor (BCR) binds to antigen that is circulating freely in the body
fluids. Involvement of a B cell in an adaptive immune response is called an
antibody-mediated immune response. This is because when
activated, B cells produce antibodies.
Antibodies are Y shaped molecules consisting of four polypeptide chains; two
identical heavy chains and two identical light chains. The end of each “arm” of
the antibody is the variable region and is the part the binds with
the antigen with a “lock and key” specificity. The variable region is unique to
only those antibodies produced from one activated B cell. Thousands of variable
regions exist. The rest of the antibody is the constant region.
 Antibody Molecule
Antibody Molecule
Different from the variable region of the antibody, only five different constant
regions exist: IgG, IgM, IgE, IgD, and IgA. The constant regions are referred to
by the Greek letters gamma, mu, epsilon, delta and alpha. When the region is on
an immunoglobulin molecule it is referred to as Ig. Therefore, immunoglobulin is
another term for antibody. These constant regions, also called
isotypes, are identical between antibodies.
Therefore every different antibody with an IgM constant region will have the same
exact IgM constant region, even though the antibodies have different antigen
specificity on its variable region. There is no variability in the constant
region. Antibodies may exist as single molecules (IgG, IgE and IgD) or may link
with another like antibody to form a dimer (IgA) or five like antibodies may
join as a pentamer (IgM). Single IgG function well to neutralize, immobilize,
clump together, or mark pathogens; dimer IgA are secreted in body fluids; and
pentamer IgM activates complement. Each immunoglobulin type is found with
different types of infection or location.
The actions of the classes of immunoglobulins differ somewhat, but all of them
act to disable antigens in some way. Actions of antibodies include the
following:
- Neutralizing antigen of some bacterial toxins and prevents attachment of
some viruses to body cells.
- Immobilizing cilia or flagella of motile bacteria thereby limiting their
spread into nearby tissues.
- Agglutinating or clumping together pathogens by antigen-antibody
cross-linking.
- Activating the classical pathway of the complement system
- Enhancing the activity of phagocytes by “marking” the pathogen as foreign
(opsonization).
- Binds to immune cells and stimulates chemical secretion
Classes of Immunoglobulins (Igs) and Their Immune Function
 Antibody Effector Functions
Antibody Effector Functions
| Isotype |
Plasma concentration and immune function |
| IgM |
5-10% Predominant antibody secreted on first exposure to antigen.
Activates complement and agglutinates pathogen |
| IgG |
80-85% Predominant antibody secreted on second exposure to antigen.
Enhances phagocytosis, neutralizes toxins, activates complement, crosses
placenta |
| IgA |
10-19% Mainly found in secretions such as tears, saliva, mucus, and
breast milk. Neutralizes and immobilizes pathogen |
| IgE |
Less than 0.1% IgE activate mast cells, basophils and eosinophils.
Involved in allergic reactions and provides protection against parasitic
infections. |
| IgD |
0.2% Function not well understood. Present on plasma membrane of B cells
and may be involved in their activation, |
Low-molecular weight proteins, called cytokines, stimulate or inhibit many cell
functions such as cell growth and differentiation. These proteins are secreted by white
blood cells and various other cells in the body such as endothelial cells, fibroblasts,
liver cells and kidney cells. The two principle regulators of the immune response are
helper T cells and macrophages. Cytokines bind to specific receptors on the membrane of
cells. Sometimes cytokines bind to receptors in the secreting cell, resulting in
self-stimulation. In general, cytokines regulate the intensity and duration of the
immune response. Cytokines affect the immune response by stimulating or inhibiting the
activation, proliferations and/or differentiation of various cells and by regulating the
secretion of antibodies and other cytokines. Some cytokines attract other immune cells
to sites of infection, promoting phagocytosis and inflammation.
There are over 200 different cytokines. Cytokines are sometimes named for the cells
producing them or for their function. Interleukins are produced by
leukocytes and lymphokines by lymphocytes. Chemokines promote
movement of cells toward chemicals (chemotaxis). Interferons interfere with
viral replication and inflammatory cytokines promote inflammation. Some
important cytokines participating in immune responses are summarized in the table
below:
| Cytokine |
Secreted by |
Target and effects |
| Interleukin 1 (IL-1) |
Monocytes, macrophages, endothelial and epithelial cells |
Vasculature (inflammation) hypothalamus (fever) |
| Interleukin 2 (IL-2) |
Helper T cells |
T and B cell proliferation; activates NK cells |
| Interleukin 4 (IL-4) |
Helper T cells |
B cell proliferation; plasma cells secrete IgE antibodies which plays a role in
allergic reactions |
| Interleukin 5 (IL-5) |
Helper T cells |
Induces eosinophil activation to help kill parasites |
| Interleukin 6 (IL-6) |
Macrophages, endothelial cells |
B cell proliferation and antibody secretion |
| Tumor necrosis factor Α (TNF-Α) |
Macrophages |
Vasculature (inflammation); neutrophil activation and fever production |
| Transforming growth factor Β (TGF-Β) |
T cells, macrophages |
Inhibits T and B cells, inhibits macrophages, promotes IgE antibodies |
| Interferon Α (IFN-Α) |
Macrophages |
Induces antiviral state in nucleated cells, increases MHC-I expression;
activates NK cells |
| Interferon Γ (IFN-Γ) |
Inflammatory T cells, Natural Killer cells |
Activates macrophages; increases antigen presentation |
| Hematopoietic growth factors |
Kidney, liver, fibroblasts, endothelial cells |
Development of red and white cells in bone marrow |
 Role of Helper T cell in Cellular Immunity
Role of Helper T cell in Cellular Immunity
Specialized immune cells that reside within the tissues and organs of the lymphatic
system recognize foreign pathogens and initiate a local and/or systemic response. These
cells are derived from a common precursor cell found in the red bone marrow called the
hematopoietic stem cell. These pluripotent hematopoietic stem cells can divide and
mature into one of a number of different types of cells. Therefore, they are termed
pluripotent, or able to produce a broad variety of cells. This means that many different
cells result from the same precursor cell. When cells divide, they mature into different
cell types in a process called differentiation. As with all stem cells, the
type of cells they differentiate into is controlled by signals in the environment where
they reside. Hematopoietic stem cells differentiate into the red blood cells
(erythrocytes), platelets and two categories of white blood cells (leukocytes), the
myeloid and lymphoid lineages. These leukocytes participate in the immune response and
further differentiate into mast-cells, granulocytes (neutrophils, eosinophils and
basophils), monocytes (macrophages), dendritic cells and lymphocytes (natural killer
cells, B-cells and T-cells)
-
Mast cells-
When an infection occurs, local immune cells react and emit signals or chemicals
to recruit other immune cells. Among the first to respond are the mast cells
that are found surrounding tissues and lymphatic vessels. When bacteria infect
tissues, mast cells recognize parts of the bacterial cell wall and release
cytokines that recruit neutrophils, eosinophils, basophils and T cells. As part
of adaptive immunity, IgE antibodies mediate release of granules, a combination
of chemicals that cause vasodilation (histamine, bradykinin), anti-coagulation
(heparin), or cell lysis (lysozymes). In this way, mast cell orchestrate
allergic responses.
-
Monocytes and Macrophages-
Monocytes circulate in the blood. Chemokines and cytokines signal for the influx
of monocytes to a specific area. The cells circulate to the location, migrate
into the tissues and differentiate into macrophages. Macrophages reside in
almost all tissues. Macrophages engulf pathogens and assist in the immune
response by presenting antigenic peptides to helper T cells. Macrophages secrete
cytokines that promote inflammation and fever.
 Interaction of Innate and Adaptive Immunity
Interaction of Innate and Adaptive Immunity
-
Neutrophils-
Neutrophils are the most abundant circulating white blood cell, comprising 50 -
70% of all infiltrating leukocytes. Tissue damage from an infection results in
the release of chemokines and other chemical attractant agents called cytokines.
These chemokines and cytokines signal for the influx of neutrophils to a
specific area. Neutrophils engulf bacteria, digest it and eliminate the
infection in a process called phagocytosis.
-
Eosinophils-
Eosinophils target parasitic infections and are abundant in allergic reactions,
although eosinophils only constitute a small percentage (1-6%) of leukocytes.
The granules of eosinophils include cytotoxic and neurotoxin proteins that can
be released to kill multicellular worm parasites. Eosinophils release their
toxic and inflammatory mediators through an adaptive immune response. Antibodies
(IgE) mediate release of their granules.
-
Basophils-
Basophils are the least abundant circulating white blood cell, constituting only
1–2% of leukocytes. Basophils are recruited by cytokines from the blood to sites
of infection. Basophils release histamine, a vasodilator, and heparin, an
anti-coagulant, that aid in the infiltration of leukocytes from the bloodstream
to site of infection. Basophils are activated through innate receptors and IgE
antibodies.
-
Dendritic cells-
Dendritic cells are large, motile cells with long, cytoplasmic extensions. There
are several kinds of dendritic cells. These cells migrate through the blood
stream from bone marrow and enter tissues. They engulf particulate matter and
continually ingest large amounts of extracellular fluid and its contents. Like
macrophages and neutrophils, they degrade the pathogens, but their main role is
not the clearance of microorganisms. Instead, encounter with pathogen stimulates
dendritic cells to activate a particular class of T lymphocytes.
 Dendritic Cell
Dendritic Cell
-
Natural killer cells-
Natural killer cells or NK cells are leukocytes that are critically important in
the lysis of viral-infected cells. It is thought that NK cells are also able to
lyse and eliminate tumor cells. Therefore, many researchers are trying to learn
how NK cells function to eliminate tumors in order to design better anti-cancer
agents. Binding of NK cells to a target cell, causes the release of granules
containing toxic substances. Some granules contain a protein called
perforin. These perforin proteins insert into the plasma
membrane of the target cell and create holes in the membrane. Extracellular
fluid flows through these channels and the cell bursts. Other granules of NK
cells release granzymes which induces the tumor or infected cell to
undergo self-destruction (apotosis). Unlike traumatic cell death (necrosis),
apoptosis is a controlled cell death that produces cell fragments that can be
quickly removed before there is damage to the surrounding cells.
 White Blood Cell Formation
White Blood Cell Formation
White Blood Cells and Their Function in Innate Immunity
| Cell |
Function |
| Mast cell |
release chemicals to initiate an immune response and orchestrate
allergic response |
| Macrophages |
phagocytosis of pathogen and release cytokines to orchestrate immune
response |
| Neutrophils |
phagocytosis of pathogen |
| Eosinophils |
destruction of worm parasites and abundant in allergic response |
| Basophils |
release chemicals to initiate an inflammatory response |
| Dendritic cells |
phagocytosis and presentation of pathogen as bridge between innate and
adaptive immune responses |
| Natural Killer cells |
release chemicals to kill virally infected cells and cancer cells |
-
B lymphocytes-
B lymphocytes (B cells) leave the red bone marrow mature and express a unique
membrane receptor (BCR) that recognizes a specific antigen. After interacting
with antigen, the B cell proliferates and these cells differentiate into
specific antibody-secreting plasma cells or memory
cells which remain waiting for the next exposure to the exact same
antigen.
 Activation and Proliferation of B Cell
Activation and Proliferation of B Cell
-
T lymphocytes-
T lymphocytes (T cells) leave the red bone marrow as pre-T cells, mature in the
thymus gland and express several distinctive proteins one of which is a membrane
receptor. This unique T-cell receptor (TCR) only recognizes a specific antigen
that is complexed with a MHC molecule. Several subpopulations of T lymphocytes
are recognized such as helper T and cytotoxic T cells.
Activated helper T cells provide signals (cytokines) that promote immune
responses. There are two subsets of helper T cells. The first subset, Th1, are
also called inflammatory T cells and recruit macrophages
to sites of infection. The second subset, Th2, are usually referred to as helper
T cells and assist B cells in antibody mediated immunity. The cytotoxic T cells
destroy target cells when activated.
-
Antigen Presenting Cells (APC)-
Dendritic cells, macrophages and B cells constitue the main APCs. These cells
present antigen to helper T cells. APCs can process and present antigenic
peptides in association with class II MHC molecules and deliver the
co-stimulatory signal necessary for T-cell activation.
 T Cell Activation
T Cell Activation
White Blood Cells and Their Main Function in Adaptive Immunity
| Cell |
Function |
| B lymphocytes (B cell) |
Recognize foreign antigen and secrete antibodies |
| T lymphocytes (T cell) |
Helper T cells provide signals that promote immune responses; cytotoxic
T cells kill target cells |
| Antigen Presenting Cell (APC) |
Present antigen to helper T cells and include dendritic cells,
macrophages and B cells |
Recall that the lymph and lymph vessels serve as a means of transporting proteins, lipids
and pathogens from intersitial fluid to the lymph tissues. Once mature lymphocytes have
been generated they circulate in the blood and migrate to lymph tissues where the
leukocytes may interact with the trapped pathogen. Lymphatic tissue surrounded by a
connective tissue capsule is said to be encapsulated and include lymph organs such as
lymph nodes, spleen and thymus. Nonencapsulated lymphatic tissue includes diffuse
lymphatic tissue, lymphatic nodules, and the tonsils. Nonencapsulated lymphatic tissue
is found in and beneath the mucous membranes lining the digestive, respiratory, urinary
and reproductive tracts. In these locations, the lymphatic tissue is strategically
located to intercept pathogens that may enter the body.
Diffuse lymphatic tissue has no clear boundary and contains dispersed
lymphocytes, macrophages, dendritic cells and other cells. It may be located deep to
mucous membranes, around lymphatic nodules and within lymph nodes and spleen.
Lymphoid nodules are denser arrangements of lymphatic tissue organized into
spherical structures, ranging in size from a few hundred microns to a few millimeters or
more in diameter. Until it is infiltrated by antigen, lymphoid nodules mainly consist of
resting B cells and antigen presenting cells (APCs). After antigenic challenge, the
nodules consist of antibody-producing B cells (plasma cells) and helper T cells
interspersed with macrophages and APCs. Many lymphatic nodules are scattered throughout
the connective tissue of mucous membranes lining the respiratory, gastrointestinal,
urinary and reproductive tracts. These lymphatic nodules are referred to as
mucosa-associated lymphatic tissue (MALT). Although lymph nodules can
occur in solitary, many occur in large aggregations. Among these are the Peyer’s
patches in the ileum of the small intestine and aggregations in the appendix.
Lymphatic nodules may also be located in the lymph nodes and the spleen. In lymph nodes.
these nodules receive the name lymphoid follicles, and they contain proliferating B
cells called germinal centers.
Tonsils are large groups of lymphatic nodules located deep to the mucous
membranes within the pharynx. They are strategically positioned forming a ring in the
pharyngeal and oral cavity where they can stimulate immune responses against inhaled or
swallowed pathogens. The adenoid is embedded in the nasopharynx, the two palatine
tonsils are on either side of the oral cavity, and the paired lingual tonsils are
located at the base of the tongue. When infected, tonsils can become inflamed. It is
possible to survive without the tonsils.
The lymphatic system consists of many different tissues and organs that are found
throughout the body. Some organs provide the environment for the development and
maturation of leukocytes. Other tissues and organs trap pathogen and are the sites where
leukocytes can interact with the pathogen. The circulatory and lymphatic systems
interact together to connect these organs and tissues.
Several morphologically and functionally diverse organs have various functions in
the development of immune responses. These can be distinguished by function as
primary and secondary lymphoid organs. The red bone marrow and thymus gland are
the primary lymphoid organs, where maturation of B and T lymphocytes occurs. It
is in the secondary lymphoid organs and tissues where the immune responses take
place. Secondary lymphoid organs include the lymph nodes and spleen.
Bone Marrow
The red bone marrow is the site where hematopoietic stem cells are found.
Hematopoietic stem cells differentiate into the red blood cells
(erythrocytes), platelets (thrombocytes) and white blood cells
(leukocytes). Leukocytes participate in the immune response and further
differentiate into mast cells, granulocytes (neutrophils, eosinophils
and basophils), monocytes (macrophages), dendritic cells and lymphocytes
(natural killer cells, B-cells and T-cells).
After birth, generation of mature B cells occurs in the bone marrow.
During B cell development, random gene rearrangement produces an
immature or pre-B cell expressing a B cell receptor (BCR). Maturation of
pre-B cells involves a positive and negative selection process. Pre-B
cells capable of an immune response survive positive selection and cells
incapable of an immune response undergo apoptosis. Negative selection
causes lymphocytes acting against self-antigens to undergo apoptosis or
stimulates them to change their receptor specificity (receptor
editing).
Thymus
The thymus is located in the chest, just in front of the heart and behind
the sternum of the rib cage. The thymus is comprised of two lobes that
are further divided into lobules. Structural thymic tissue consists of
epithelial cells joined by desmosomes. This framework of cells form
small, irregularly shaped compartments filled with lymphocytes. In
addition, the network of epithelial cells surrounds capillaries to form
a blood-thymus barrier which prevents the entry of foreign antigens into
the thymus. Each lobule consists of an outer cortex and an inner
medulla. Immature, pre-T cells (thymocytes) migrate from the red bone
marrow to the cortex of the thymus where they mature. Thymocytes pass
through a series of stages maked by changes in expression of the TCR and
cell-surface proteins such as CD4 and CD8. Epithelial cells in the
thymus assist in the maturation process of T cells in the thymic cortex
in a process called positive selection. In this process
immature T cells express T-cell receptors (TCRs) that interact with
class I MHC complexed with self proteins on epithelial cells in the
cortex. Cells that recognize the MHC part of the complex survive due to
self-recognition. T cells that do
not recognize self MHC undergo apoptosis. At the junction of
the cortex and the medulla, the development of
self-tolerance occurs. The development of
self-tolerance occurs in a process called negative selection
in which the T cells interact with dendritic cells presenting
class I MHC complexed with self proteins. T cells whose TCRs recognize
the MHC and also recognize self proteins undergo
apoptosis. This process is referred to as clonal deletion.
In this way the surviving T cells recognize self MHC (self-recognition)
and lack reactivity to self proteins (self-tolerance). The surviving T
cells enter the medulla of the thymus and eventually leave the thymus
via the blood and migrate to lymph nodes, the spleen and other lymphatic
tissues where they colonize parts of these organs. Functionally the
thymus continues to mature until puberty. As a person ages the funtional
portion of the thymus gland atrophies considerably. However, before the
thymus atrophies, it populates the secondary lymphatic organs and
tissues with T cells. Like the spleen and tonsils, it is possible to
survive without a thymus since the secondary lymphatic organs have
already been populated with mature T cells, but the ability to initiate
an immune response is diminished.
Lymph Nodes
As the lymph fluid moves from the capillary beds through the lymphatic
vessels, and before it flows back into the general circulation it enters
into areas of small swellings in the vessels called lymph nodes. Lymph
nodes are oval shaped structures that are enclosed in a fibrous outer
capsule. Superficial lymph nodes are in the subcutaneous tissue and
include inguinal nodes in the groin, axillary nodes in the armpit and
cervical nodes in the neck. Deep lymph nodes are everywhere else in the
body where there are lymph vessels. Nodes collect and filter lymph from
several afferent vessels, and several efferent vessels carry the lymph
out from the node. The lymph fluid enters the lymph node and slowly
filters through the cortex and medullary regions. Any microbes that were
collected in the lymph are detected by the immune cells in the lymph
nodes.
Lymph nodes are primarily a site for immune system activation.
Specialized immune cells, including B lymphocytes, T lymphocytes, and
macarophages, leave the bone marrow or thymus gland and reside within
the lymph node. Just inside the fibrous capsule is the outer cortex
region that contains the lymphatic nodules, a place where the B
lymphocytes encounter antigen and the product of B cells, called
antibodies, are generated. Memory B cells persist after an initial
encounter in the lymphatic node. The inner cortex (paracortex) contains
T cells and dendritic cells that enter a lymph node from other tissues.
The dendritic cells present antigens associated with MHC class II to
Helper T cells causing their proliferation. At the interior of the lymph
node is the medulla, where antibody producing B cells (called plasma
cells) are located. Antibodies leave the lymph nodes via the
lymph.
Spleen
In addition to lymph nodes, the lymphatic system includes the spleen. The
spleen is located in the left, superior corner of the abdominal cavity,
just posterior to the stomach, and protected by the rib cage. Although
it is about the size of a clenched fist, it is one of the largest immune
organ. Shaped like a big lymph node, the spleen similarly contains
macrophages and lymphocytes. Instead of lymph flowing through it, the
spleen contains blood. In fact, it serves as a reservoir for blood which
can be used for a small increase in circulating red blood cells during
exercise or emergency situations. Splenic macrophages can phagocytize
cellular debris from defective red blood cells. Foreign substances in
the blood passing through the spleen can stimulate an immune response
because of the presence of lymphocytes.
The spleen consists of two different kinds of tissue called white pulp
and red pulp. Blood flowing into the spleen through the splenic arteries
enters the central arteries of the white pulp. Within this pulp, B and T
cells carry out immune functions similar to lymph nodes. White pulp also
contains macrophages that can remove blood-borne pathogens by
phagocytosis. Within the red pulp, macrophages can remove worn-out red
blood cells and platelets.
It is possible to survive without a spleen if it is damaged. However,
removal of the spleen does diminish the ability to mount an immune
response.
Overview of the Immune System
As you have learned, the lymphatic system and immunity are closely related.
Immune responses originate in the lymph organs and tissues. The immune system
constantly surveys our body for invading bacteria, viruses, or other foreign
pathogens. When it encounters a foreign substance, the immune system is
activated to destroy the pathogen. Although fully integrated, the immune
response is divided into two categories: the innate immune response and the
adaptive immune response. The innate immune response is activated immediately
(within hours to days) and is always the same. It responds to non-specific cues
from the invading pathogens like highly conserved protein peptides that are
expressed by many different types of pathogens, and are not expressed on
mammalian cells. For example, each time a bacterial cell enters the body it is
recognized and phagocytized with the same speed and efficiency. The innate
immune response can be so efficient that signs or symptoms of disease never
develop.
The adaptive immune response is slow (days to weeks) on first exposure to
pathogens, but improves with subsequent exposure. On first exposure, cells of
the adaptive immune response identifies a specific pathogen based on the
specific proteins in that pathogen and mounts a targeted response to eliminate
it. This type of response takes longer to establish and damaged tissues produce
signs and symptoms of disease. However, during this time the most important
aspect of our adaptive immune response is that it has memory, also called
immunological memory. The adaptive immune response produces memory cells so that
it is able to recognize foreign substances it has seen before and eliminate the
threat both faster and with greater efficiency. Consequently, no signs or
symptoms of disease develop and an immunity to that pathogen now exists.
Vaccines have been created to take advantage of the immune memory. Because of
this, the immune system can be used to prevent many infections and to ward off
many diseases, including cancers.
 Primary Immune Response
Primary Immune Response
Immunity can be seen as a state of protection from infectious disease and has both a less
specific innate response and more specific adaptive response. Innate and adaptive
immunity are intimately linked. The less specific component provides the first line of
defense against infection, most of which fall into the category of innate immunity.
Barriers, antimicrobial compounds and phagocytic cells all play important roles in
innate immunity and are uniform in all members of a species.
Innate immunity includes mechanisms that are not specific to a particular pathogen but
include molecular and cellular components that recognize classes of molecules peculiar
to frequently encountered pathogens.
Innate immunity can be seen to comprise five types of defensive mechanisms:
- anatomic barriers
- physiologic barriers
- phagocytosis
- inflammation
- fever
 Innate Defense System
Innate Defense System
Anatomical and Physiological Barriers
Our immune system is constantly preventing infection by potential invaders. The
vast majority of the time we are unaware of any potential threat. The first
lines of defense our bodies have against infection are the physical and
anatomical barriers. Most infections are avoided because the pathogen cannot
penetrate the barriers in place in our body.
The Skin
The largest of the anatomical barriers is our skin. Our skin not only covers and
protects the internal organs, it also acts as a shield to prevent infection from
invading foreign pathogens. Two layers of cells make up the skin; the innermost
layer or dermis, and the outermost layer or epidermis.
Within these layers are sweat glands, hair follicles, nerves, and blood vessels
that collectively serve to prevent entry into the body, to eliminate excess heat
from the body, and to inform the body about the external environment.
Invading pathogens cannot penetrate the epidermal layer of the skin. This makes
the skin an incredibly important organ involved with the immune response. Only
if the integrity of the skin is broken or damaged can pathogens gain entry.
Further, this is only when the wounds are open. Once wound repair has begun and
scabbing has occurred, the skin returns to its impenetrable state.
The Brain
Unlike the other organs of the body, the brain does not contain lymphatic
vessels. Therefore, the brain lacks a system to filter out invading pathogens.
Instead, a physical barrier between the brain and the rest of the body exists.
To prevent infection, subset of glial cells in the brain called astrocytes
surround the capillaries in the brain to slow or prevent the movement of
some proteins and pathogens from the blood into the brain. These cells add
another layer that molecules or pathogens have to cross in order to gain access
into the brain, called the blood-brain barrier. Because of their
size and the thickness of the cell layer, pathogens can rarely cross this
barrier. Therefore the brain is protected from infection even though it lacks a
lymphatic system. Due to the presence of the blood-brain barrier, the brain is
considered an immunologically privileged organ. This means that only certain
proteins can gain access to the brain. Sometimes even beneficial medications
cannot cross the blood brain barrier.
Physiological Barriers
Potentially infectious pathogens can gain access into the body through any of the
openings, including the eyes, ears, nose, mouth, lungs, anus, vagina, or urethra
openings. To avoid infection, each of these openings has mechanisms in place to
prevent entry. For example, the eyes are protected by the eye lashes, the lid,
fluid around the eye, and by tears. Tears not only flush potentially infectious
pathogens from the eye, they also contain anti-microbial proteins such as
lysozyme.
Ears have small hairs and produce ear wax to trap and remove pathogens. Likewise,
nasal passages contain hair to trap pathogens. Nerve endings in the nose can be
stimulated by foreign particles, resulting in the sneeze reflex that forcibly
blows pathogens from the nasal opening. The mouth secretes lysozymes in response
to foreign pathogens. Lysozyme destroys the bacterial cell wall and kills the
invading bacteria. If a pathogen successfully evades the barriers and passes
through the mouth or nose, it can be trapped in the mucus membrane that lines
the lungs. Small microtubules, called cilia, sweep back and forth in a whip-like
motion to push the mucus up and out of the lung. This is referred to as the
ciliary escalator and helps remove small particles, smoke particulates, and
pathogens from the lung. Mucus that exits the lung is usually swallowed and
enters the esophagus.
Mucus, tight sphincters, and secretions with low pH reside within the openings of
the urogenital tract that prevent infection and kill invading pathogens by
physically blocking, trapping, or lysing the cells.
Other physiological events help remove potentially harmful pathogens including
vomiting, coughing, diarrhea, and urinating. Because of the high or low pH, and
force and speed of expulsion, these mechanisms help flush the body of harmful
agents quickly and prevent digestion or absorption through the GI or respiratory
system.
Anatomical and Physiological Barriers that Help Prevent Infection
| Site |
Factor |
Function |
| Eye |
tears |
flushing pathogens from the eye |
| Eye |
lysozyme |
lysing bacterial cell walls |
| Eye |
lashes |
trap pathogens |
| Eye |
lid |
prevent entry |
| Skin |
sweat |
flushing pathogens from the skin |
| Skin |
hair |
trap pathogens |
| Mouth |
saliva |
trap pathogens |
| Mouth |
lysozyme |
lysing bacterial cell walls |
| Nose |
hair |
trap pathogens |
| Nose |
sneeze |
forcefully expel pathogen |
| Lungs |
mucus |
trap pathogens |
| Lungs |
cilia |
push pathogen-containing mucus out of the lungs |
| Lungs |
cough |
forcefully expel pathogen |
| Stomach |
hydrochloric acid |
create low pH to lyse cells |
| Stomach |
vomit |
expel pathogens quickly |
| Intestine |
mucus |
trap pathogens |
| Intestine |
diarrhea |
expel pathogens quickly |
| Vagina |
mucus |
low pH that lyses pathogen |
Many of the immune cells involved in innate immunity have the property of pattern
recognition, the ability to recognize a given class of molecules. Some types of
molecules are unique to microbes and never found in humans. A strong feature of innate
immunity is the ability to immediately recognize that a foreign molecule is in the body
and rapidly remove it. Immune cells recognize specific molecules on the surface of
foreign pathogens called pathogen-associated molecular patterns (PAMP).
These PAMPs are common to groups of pathogens, but are not found in human cells.
Pattern recognition receptors (PRR) found on the surface of the immune
cell bind the PAMPs and initiate the innate immune response. Toll-like receptors (TLRs) are the most important family of innate immunity receptors.
Both innate and adaptive immune responses depend on responses of white blood cells. These
cells all originate in the bone marrow. There are two main lineages of leukocytes, the
myeloid and lymphoid. Most of the cells of innate immunity
are derived from the myeloid lineage. The common myeloid progenitor is the precursor of
the macrophages (the mature form of the monocyte), mast cells, dendritic cells and
granulocytes. Granulocytes can further differentiate into neutrophils, eosinophils, and
basophils. Collectively, these cells are referred to as the inflammatory
cells. Influx of these cells into an infected area produces inflammation that
is characterized by redness, heat, pain and swelling.
When an infection occurs, local immune cells react and emit signals or chemicals to
recruit other immune cells. Among the first to respond are the nonmotile mast cells that
are found near capillaries in the connective tissue of the skin, lungs, gastrointestinal
tract and urogenital tract. Through PPRs, mast cells are stimulated to release cytokines
that recruit neutrophils, eosinophils, basophils and T cells.
Example
Local Immune Cell Reaction
When bacteria infects the body, mast cells recognize parts of the bacterial cell wall
and release a combination of chemicals including histamine, heparin, serotonin,
bradykinin, and lysozymes that cause vasodilation (histamine, bradykinin), blood
thinning or anti-coagulation (heparin), or cell lysis (lysozymes). Together, these
chemicals increase the permeability of the capillaries to allow for other immune
cells to infiltrate the extracellular space of the local infected region.
In addition to mast cells, other specialized immune cells including a group of cells,
called the granulocytes, respond to infection. Granulocytes include
neutrophils, eosinophils, and basophils.
Neutrophils are the most abundant white blood cell, comprising 50 - 70% of all
infiltrating leukocytes. Neutrophils are usually the first cells to leave the blood and
enter infected tissues in large numbers. They are especially effective in engulfing and
digesting bacteria as a way of combating infections. Neutrophils are programmed to die
by apoptosis after ingesting bacteria. Pus is an accumulation of dead
neutrophils, dead pathogens, tissue debris and fluid. Eosinophils target parasitic
infections and are abundant in allergic reactions, although eosinophils only constitute
a small percentage (1-6%) of leukocytes. Eosinophils release chemical factors called
chemokines. These are chemicals that attract other immune cells and
initiate an adaptive immune response. Basophils are the least abundant, constituting
only 1–2% of leukocytes. Basophils release histamine, a vasodilator, and heparin, an
anti-coagulant, that aid in the infiltration of leukocytes from the bloodstream to site
of infection.
Monocytes circulate in the blood and continually migrate into tissues where
they mature into macrophages. Macrophages are large motile cells that leave the blood
and accumulate in infected tissues after neutrophils. They outlive neutrophils and can
ingest more and larger substances than neutrophils can. They are responsible for most of
the activity in the late stages of an infection, including the cleanup of dead
neutrophils and general debris. Macrophages are also permanent residents beneath the
free surfaces of the body and around blood and lymphatic vessels. In these locations
macrophages provide protection by trapping and destroying pathogens entering the
tissues. Macrophages are waiting within the lymph nodes, spleen, bone marrow and liver
as well. An additional role of macrophages is to coordinate immune responses: they
induce inflammation, which we will see is a prerequisite to a successful immune
response, and they secrete cytokines that activate and recruit other immune system cells
into an immune response. Although macrophages can activate naïve T cells through antigen
presentation, they often present antigens to T cells to be activated themselves. Some
microorganisms, such as mycobacteria, are intracellular pathogens that grow primarily in
the phagosomes of macrophages. Peptides derived from such microorganisms can be
presented on the macrophage surface by MHC class II molecules. These complexes are
recognized by the T-cell receptor of the TH1 subset. The T cells synthesize
molecules that stimulate the macrophage and enable it to eliminate the pathogen. This
antimicrobial boost to the macrophage is known as macrophage activation.
This coordination between T cells and macrophages results in the formation of the immune
reaction call granuloma, in which microbes are held at bay within a central
area of macrophages surrounded by activated T lymphocytes.
Dendritic cells have almost all of the innate immunity receptors and are extremely
sensitive to the presence of pathogens. Stimulation of innate immunity receptors
promotes the ingestion and digestion of the pathogen. As part of innate immunity,
dendritic cells produce cytokines that attract other immune cells to sites of infection
and interferons that inhibit viral infection. Dendritic cells in the skin are called
Langerhans cells.
The lymphoid progenitor in the bone marrow gives rise to the antigen-specific lymphocytes
of the adaptive immune system and also a type of lymphocyte that responds to the
presence of infection but is not specific for antigen, and so it is considered to be
part of the innate immune system. These large cells have distinctive granular cytoplasm
and are called a natural killer cells (NK cells). These cells
recognize and kill some abnormal cells, such as tumor cells and cells infected with
viruses, and are thought to be important in controlling viral infections until the
adaptive immune response has a chance to kick in.
Phagocytosis
Phagocytes are specialized cells that engulf and ingest microbes or other
particles such as cellular debris. This process is termed
phagocytosis. The two main types of phagocytes are neutrophils
and macrophages. When an infection occurs, these cells migrate to the infected
area. During this migration macrophages enlarge and develop into wandering
macrophages. Fixed macrophages stand guard in specific tissues; such as
histocytes in connective tissue, Kuppfer cells in the liver and tissue
macrophages in the spleen and lymph nodes. In addition to being an innate
defense mechanism, phagocytosis by macrophages and dendritic cells plays a vital
role in antigen presentation in adaptive immunity.
As part of innate immunity, phagocytosis occurs in four phases:
-
Chemotaxis. Chemicals from invading microbes, damaged tissue cells,
leukotrienes from basophils and mast cells or activated complement proteins
stimulate movement of phagocytes to a site of damage.
-
Adherence. Phagocyte attaches to the microbe or other foreign material.
Leukotrienes, complement proteins and antibodies bound to the pathogen will
enhance adherence (opsonization).
-
Ingestion. Plasma membrane extensions (pseudopods) of the phagocyte would
engulf the microbe, surround it and internalize the microbe in a sac called
a phagosome.
-
Digestion. In the cytoplasm the phagosome and vesicles filled with digestive
enzymes and lethal oxidants called lysosomes fuse into a larger vesicle
called a phagolysosome. This chemical onslaught quickly kills many
microbes.
 Phagocytosis
Phagocytosis
Inflammation
When pathogens breach the epithelial barrier and enter a tissue, they encounter
the resident mast cell, macrophages and dendritic cells. The macrophages and
dendritic cells phagocytize the pathogen. The macrophage and mast cells release
cytokines that attract neutrophils to the site of infection. This is the
beginning of a complex innate immune response called inflammation. Inflammation
results in the activation and accumulation of immune cells, the release of
inflammatory chemicals and the activation of complement.
The four characteristic or cardinal signs and symptoms of inflammation are
redness, heat, swelling and pain. Loss of function may also occur at the site
depending on the extent of the injury. Inflammation is an attempt to restore
tissue homeostasis by removing microbes, toxins or debris at the site of injury
and to prepare the site for repair. Inflammation is a normal response to tissue
damage and a vital part of tissue repair.
Whether the injury was due to abrasion, burns or viral infection, the
inflammatory response has three basic stages:
- Vasodilation and increased permeability of blood vessels. Vasodilation is an
increase in blood vessel diameter that allows more blood to flow to the site
of injury. Increased permeability permits proteins such as antibodies,
clotting factors and complement to enter the injured area because they can
leak out of the capillaries.
- Emigration of phagocytes. Emigration is the movement of phagocytes from the
blood into interstitial fluid. Phagocytes remove pathogens and dead
tissue.
- Tissue repair. In response to injury, mast cells in connective tissue and
basophils in blood release histamine. Neutrophils and macrophages attracted
to the site of injury also stimulate the release of histamine, which causes
vasodilation and increased permeability of blood vessels. Leukotrienes (LTs)
produced by basophils and mast cells increase permeability and are
chemotactic agents that stimulate emigration. Other substances that
contribute to vasodilation and increased permeability are blood kinins and
complement components and prostaglandins released by damaged cells.
Prostaglandins also intensify and prolong the pain associated with
inflammation.
 Inflammation
Inflammation
White Blood Cells and their Functions in Innate Immunity
| Cell |
Function |
| Mast cell |
release chemicals to initiate a allergic inflammation |
| Neutrophils |
phagocytosis of infected cells and bacteria |
| Eosinophils |
destruction of parasites |
| Basophils |
release chemicals to initiate a allergic inflammation |
| Natural Killer cells |
release chemicals to kill tumor or virally infected cells |
| Macrophages |
phagocytosis and orchestration of immune responses |
| Dendritic cells |
phagocytosis and antigen presentation |
The Kinin-Kallikrein and Complement Systems
There are two additional systems in the body that aid the inflammatory response
to eliminate infection: the kinin-kallikrein and the complement systems. These
are the non-specific mechanisms that are part of the innate immune response.
The Kinin-Kallikrein System
When tissues and cells are damaged, the interior components of their cells are
released to the external environment. These components are not found outside
cells normally, and are therefore recognized by the innate immune response.
Specifically, the release of intracellular proteins from damaged tissue
stimulates the activation of the kinin-kallikrein system. As a result, a cascade
of proteins is activated that ultimately produce a chemical called bradykinin.
Within tissues, bradykinin serves as a vasodilator that increases the
permeability of the blood vessels. This increased permeability allows the larger
leukocytes to leave the blood vessels and enter into the infected area.
The Complement System
The second system activated in response to local inflammation is the complement
system. This system can be activated through three different pathways: the
classical, alternative, and the MB/Lectin pathways. Activation of any of these
pathways results in the creation of a membrane attack complex or MAC. The MAC is
made up of 9 proteins that assemble in the presence of an infected cell. They
form a complex that can bind to the surface of an invading pathogen or infected
cell. MACs are a part of the innate immune response that non-specifically
recognize pathogen proteins and bind to the cell surface. When bound, the MAC
pokes holes in the membrane of the pathogen and lyses it. Therefore, assembly
and activation of the MAC destroys an invading pathogen.
 Complement
Complement
 Effector Function of Complement Proteins
Effector Function of Complement Proteins
Fever
Fevers usually occur in response to infection or inflammation. Fever is an
elevated core body temperature (37.2-37.5 C or 99.0-99.5 F in adults) that
occurs because a thermostat in the hypothalamus is reset to a higher setpoint.
Many bacterial toxins can elevate body temperature by triggering release of
fever-causing cytokines (pyrogens) such as interleukin-1 from macrophages.
Positive benefits of this elevated body temperature include, inhibiting the
growth of some microbes and speeding up chemical reactions involved in the
immune response.
Review of Innate Immunity
The innate immune response system produces a non-specific response to invading
pathogens. Immune cells, such as mast cells, macrophages and dendritic cells,
recognize protein patterns called pathogen-associated molecular patterns (PAMPs)
on the surface of pathogens and mount a localized immune response. Local immune
cells release chemical mediators, including chemokines, interferon, and other
chemicals that increase the permeability of the local capillaries. These
chemicals attract leukocytes, such as neutrophils circulating in the blood, and
facilitates their transport across the cell membrane into the tissue. As
leukocytes enter the infected area, localized swelling, pain, redness, and heat
are produced. These are the hallmark signs of inflammation and may also lead to
immobilization of loss of function. Macrophages are also recruited. These are
phagocytes that engulf pathogenic organisms, in essence eating the invading
pathogens. In addition, non-specific signaling cascades, the kinin-kallikrein
and the complement system are activated. These produce bradykinin or other
chemical mediators to facilitate influx of leukocytes and assemble the membrane
attack complex (MAC) that punches holes in the membrane of the pathogen to lyse
the cells.
 Primary Immune Response
Primary Immune Response
Recall that immune cells that are part of the innate immune response have receptors that
recognize molecules on the surface of invading pathogens that are common to many
different pathogens. Since they are common, they also have not significantly changed
over thousands to millions of years. Some pathogens, however, have developed mechanisms
to protect the molecules on their outer membranes and shield them from the immune
system. For example, some bacteria and viruses have added an extra layer to their outer
protein coat. This coating helps the pathogen evade detection by the phagocytic cells of
the innate immune system.
Adaptive responses are initiated when antigen and antigen-presenting cells, particularly
dendritic cells presenting antigens picked up at sites of infection, enter into
secondary lymphoid tissue. Dendritic cells have pattern recognition receptors that
recognize molecular patterns common to microorganisms. Binding to receptors stimulates
dendritic cells to engulf and degrade the microbe intracellularly. Dendritic cells can
also take up extracellular material, including viruses and bacteria in a manner that is
receptor-independent. The dendritic cells then migrate to secondary lymph tissue and
present this antigen to T lymphocytes. This process will be discussed in more detail in
a later module. Free antigen can also stimulate antigen receptors of B cells, but most B
cells require help from activated helper T cells. The activation of naïve T lymphocytes
is therefore an essential first step in adaptive immune responses. For the adaptive
immune response to occur, naïve lymphocytes first have to recognize antigens. After
recognition, the naïve lymphocytes divide and differentiate, producing a large number of
lymphocytes capable of responding to the antigen.
 Comparison of Antibody Isotype Titer in Primary and Secondary Immune
Response
Comparison of Antibody Isotype Titer in Primary and Secondary Immune
Response
The innate and adaptive immune responses have two important differences. First is time.
Recall that most components of innate immunity are present before the onset of
infection. In contrast, adaptive immunity does not come into play until after the onset
of infection. Adaptive immunity responds with a high degree of specificity. Because the
adaptive immune response is initiated in response to the recognition of a specific part
of a pathogen, it takes 7-10 days to mount an effective response. Contrast this to an
innate immune response that is established immediately, and enhanced within minutes to
hours. The second difference is the creation of immunological memory. Adaptive immune
responses remember that a specific pathogen has been detected before. When the pathogen
is encountered again, the immune response that is elicited is both faster and more
robust.
T cells, B cells, and Clonal Expansion
T and B lymphocytes are the mediators of the adaptive immune
response. T and B
cells are able to identify a specific pathogen and initiate an
immune response.
Any molecule that can bind specifically to an antibody or generate
peptide
fragments that are recognized by a T-cell receptor is called an
antigen. Lymphocytes do not recognize an entire antigen. Antigenic
determinants, or epitopes, are specific regions of a given antigen
to which an
antibody or antigen receptor will bind. Because antigens are larger
molecules a
variety of epitopes could be found on a single antigen. Each T and B
cell
recognizes only one epitope. All the lymphocytes of a given clone
have identical
antigen receptors, which combine with a specific epitope. This is
like a lock
and key system. The pathogen has a protein that is broken down into
a protein
fragment (peptide). This peptide is the epitope that acts like a
key that only
fits one lock. That lock is the receptor on the T or B cell. When
the epitope
(key) is inserted into the matching T cell receptor or B cell
receptor (lock) it
initiates a series of events (turning the key) which activates the
immune
response that will affect the microbe expressing that one epitope.
Because T and
B cells only recognize one antigen, each T and B cell is said to be
antigen-specific. In order to recognize all the different antigens,
thousands
and thousands of different T and B cells are created and circulate
in our
bodies. If a T or B cell encounters its target pathogen, then that T
or B cell
becomes stimulated and begins to rapidly divide. Each mitotic
division produces
an exact copy of the parent cell (clone). Thousands of clones are
created in a
process called clonal expansion. This produces thousands of
daughter cells that
all have receptors specific for the same epitope. Adaptive immunity
occurs later
than innate immunity because the few B cells and T cells specific
for the
pathogen must first undergo clonal expansion before they
differentiate into
effector cells and migrate to the site of infection.
A naïve lymphocyte is a mature helper T cell, cytotoxic T cell or B cell that has
not yet bound to the epitope to which its receptor is specific. Naïve
lymphocytes continually pass through peripheral lymphatic tissues where they may
encounter an antigen. For example a naïve lymphocytes can pass from one lymph
node to blood and be carried to another peripheral lymhatic tissue. Naïve T and
B cells continuously circulate in the blood, the tissues, and the lymph vessels
and nodes. When they encounter and recognize a pathogen within the lymph system,
they stop circulating and begin to clonally expand.
 T Cell Activation and Clonal Expansion
T Cell Activation and Clonal Expansion
Antigens are brought from infected tissues to peripheral lymphatic tissues by dendritic
cells, which capture antigens in two ways. Stimulation of innate immunity receptors on
the dendritic cell by the pathogen promotes phagocytosis by the dendritic cell and the
display of antigens with MHC class II molecules. It is also possible that the dendritic
cell is infected by an intracellular pathogen, such as a virus, which results in antigen
display by MHC class I molecules. In response to the antigen display with MHC, dendritic
cells become mobile and move along with lymph to the nearest lymphatic tissue, where
they present the antigen to naïve lymphocytes.
Antigens in lymph and blood can also enter peripheral lymphatic tissues and stimulate the
innate immunity receptors of macrophages, which phagocytize the antigens and display
epitopes with MHC class II molecules.
Dendritic cells and macrophages expose naïve T cells to their epitopes, resulting in
their activation. Activated helper T cells assist in the activation of B cells and
cytotoxic T cells.
 Activation and Proliferation of B Cell
Activation and Proliferation of B Cell
Antigen Processing and Presentation
T cells only recognize fragments of antigenic proteins that are processed and
presented by specific immune cells. In antigenic processing, antigenic proteins
are broken down into peptide fragments that then associate with MHC molecules
. Next
the MHC:epitope complex is inserted into the plasma membrane of a body cell. The
insertion of the complex into the plasma membrane is called antigen
presentation. Antigen processing and presentation occurs in two ways depending
on whether the antigen is located intracellular or extracellular.
Processing of Exogenous Antigens
Foreign antigens present in fluids outside body cells are termed exogenous
antigens. Examples include intruders such as bacteria and bacterial toxins,
viruses that have not yet infected a cell, parasitic worms, and inhaled pollens.
Antigen-presenting cells (APCs) process and present exogenous antigens. They are
located in places where antigens are likely to penetrate the innate barriers and
enter the body. Such places include the the skin (for example, Langerhans cells
are dendritic cells) and mucous membranes that line the respiratory,
gastrointestinal, urinary and reproductive tracts. After processing antigen, APCs
migrate from tissues via lymphatic vessels to lymph nodes where they will
present their MHC:epitope complex to T lymphocytes.
The steps in the processing and presenting an exogenous antigen by an APC occur
as follows:
- Ingestion of the antigen by phagocytosis or endocytosis.
- Digestion of antigen into peptide fragments within the phagosome or endosome
by protein-digesting enzymes.
- Synthesis of class II MHC molecules (MHC-II) in the endoplasmic
reticulum.
- Packaging of MHC-II molecules into vesicles by the Golgi complex.
- Fusion of the phagosome or endosome containing the antigen fragments with
the vesicle containing the MHC-II.
- Binding of the peptide fragment to MHC-ll molecules.
- Insertion of the MHC-II/epitope complex into the plasma membrane of the APC
by exocytosis.
It is important to note that exogenous antigens are always enclosed in vesicles
when they are brought into the cell. In this way normal cellular peptides are
not complexed with class II MHC, only foreign peptides. After processing an
antigen, the APC migrates to lymphtic tissue to present the antigen to helper T
cells. This informs T cells that specific intruders are present in the body and
that immune responses should begin.
Processing of Endogenous Antigens
Antigens that are present inside the body cells are called endogenous antigens.
All nucleated cells of the body have the ability to present antigens complexed
with a class I MHC molecule. When a peptide fragment comes from a self-protein,
T cells ignore the MHC-I/epitope complex. However, if the peptide fragment comes
from a foreign protein, T cells recognize the MHC-I/epitope complex and an
immune response occurs. Such foreign antigens may be viral proteins produced
after a virus infects the cell and takes over the cell's metabolic machinery,
toxins produced from intracellular bacteria, or abnormal proteins synthesized by
a cancerous cell.
The steps in the processing and presenting of an endogenous antigen by nucleated
cell.
- Intracellular proteins are degraded into short peptides by a proteasome. A
proteosome is a large protein-enzyme complex.
- The peptide fragment is transported to the rough endoplasmic reticulum
(RER).
- MHC-I molecule is made in the RER.
- In the RER the MHC-I captures the peptide fragment.
- MHC-I/epitope complex is transported from RER to the Golgi complex.
- Insertion of the MHC-I/epitope complex into the plasma membrane of the
nucleated cell by exocytosis.
Note the difference in antigen processing between exogenous and endogenous
peptides. Exogenous peptides and MHC-II complex together in vesicles within the
cell. Endogenous peptides complex with MHC-I in the RER in the cell. Most cells
of the body can process and present endogenous antigens. The display of an
endogenous self-antigen bound to an MHC-I molecule signals that a cell is
normal, however, an endogenous foreign antigen bound to a MHC-I signals that a
cell has been infected or is abnormal and needs attention. Cytotoxic T cells
have receptors for foreign MHC-I/epitope complex.
Activation of T Cells and Immune Responses
T lymphocytes come in several varieties. They are helper T (Th) cells and
cytotoxic T cells (Tc). Each type plays an important role in the adaptive immune
response. Helper T cells bind to cells and release cytokines needed for
coordinating virtually all immune responses. Cytotoxic T cells bind to cells and
lyse them to destroy them. There are several subsets of cytotoxic and helper T
cells that employ different techniques to recognize and eliminate pathogens.
Ultimately T cells all function to specifically recognize and destroy pathogens
associated with a self-cell and by recruitment of immune cells, hence the term
cell-mediated responses.
Most mature T cells are naïve because they have not yet encountered a pathogen.
However, when an antigen enters the body only a select few T cells have T-cell
receptors (TCRs) that can recognize and bind to that specific antigen. Recall
that TCRs recognize and bind only to foreign antigen fragments that are
presented in MHC/epitope complex. Antigen recognition also involves other
surface proteins on T cells. Helper T cells have TCR and CD4 glycoproteins that
recognize class II MHC on antigen presenting cells (APCs). Cytotoxic T cells
have TCR and CD8 glycoproteins that recognize class I MHC found on all nucleated
cells of the body (target cells). These CD4 and CD8 glycoproteins help maintain
the TCR-MHC/epitope coupling and direct the appropriate immune response. For
this reason they are referred as coreceptors. Antigen/MHC recognition by a TCR
with CD4 or CD8 glycoprotein is the first signal in
activation of a T cell.
A T cell becomes activated only if it receives the first signal described
previously and at the same time receives a second
signal, a process called costimulation. Molecules involved in
costimulation include cytokines such as IL-2 and pairs of plasma membrane
molecules on the surfaces of the T cell and on the APC. One of the most
important costimulators is B7 on APCs, which binds to CD28 on helper T cells. A
resting APC expresses very little B7, but after activation by antigen, the APC
expressess B7 and increase the MHC/epitope complexes on its surface.
The two signals essential to activate a T cell are similar to starting a car
engine and putting it into gear. The correct key (epitope) in the ignition (TCR)
turns it on but it will not move forward unless you move the gear shift into
drive (costimulation). The need for costimulation helps prevent the immune
response from occurring inappropriately. Once a T cell has received these two
signals it is activated. An activated T cell then undergoes
proliferation and differentiation in a process called clonal
selection.
Inactive helper T cells recognize exogenous antigen fragments associated with
class II MHC molecules on the surface of APCs. With the aid of the CD4 proteins,
a subset of helper T cells becomes activated. Once activated the CD4+helper T
cell undergoes mitotic divisions forming identical copies of itself (clones).
This proliferation and differentiation of T helper cells produces both active
helper T cells and memory helper T cells. Within hours of activation, active T
helper cells start secreting cytokines. Cytokines secreted by these helper T
cells act as costimulators for resting helper T cells or cytotoxic T cells, and
these cytokines also enhance activation and proliferation of B cells,
macrophages and natural killer cells. Memory helper T cells are not
active during this current immune response, however, if the same antigen enters
the body again, many memory helper T cells can rapidly proliferate and
differentiate into active helper T cells and memory helper T cells.
 Activation and Dfferentiation of Helper T Cell
Activation and Dfferentiation of Helper T Cell
Most T cells that display CD8 glycoproteins develop into cytotoxic T cells.
Cytotoxic T cells are most effective against pathogens that live inside the
cells of the body. Cytotoxic T cells recognize endogenous antigen fragments
associated with class I MHC molecules displayed on the surface of nucleated
cells (target cells) infected with a pathogen, some tumor cells displaying tumor
antigens or cells of a tissue transplant.
Only dendritic cells, the most effective APCs, provide sufficient stimulation to
activate naïve cytyotoxic T cells. Dendritic cells acquire antigen by being
infected or through phagocytosis of an infected cell. The antigen is processed
and a class I MHC molecule presents the epitope to a naïve cytotoxic T cell.
Recognition requires TCR-MHC-I/epitope and CD8 coupling. In addition to antigen
recognition (signal 1), costimulation occurs when IL-2 and when B7 combines with
CD28 (signal 2). Once activated, cytotoxic T cells undergo clonal expansion. The
cytotoxic T cell divides, resulting in an increased number of cytotoxic T cells.
Most of the T cells are fully functional cytotoxic T cells that are capable of
killing infected cells. However, a small percentage of these T cells are memory
T cells, which can produce a secondary immune response on subsequent exposure to
this pathogen.
 Effector Function of Cytotoxic T Cell
Effector Function of Cytotoxic T Cell
Stimulation by dendritic cells alone can be insufficient to activate naïve
cytotoxic T cells. Helper T cells can provide assistance when they recognize
MHC-II/epitope displayed on the same dendritic cell that is activating the
cytotoxic T cell. The helper T cell stimulates the dendritic cell to express
more B7 molecules, which promotes costimulation. In addition, the helper cell
secretes IL-2, which promotes proliferation and differentiation of the cytotoxic
T cell.
 Killing of Target Cell by Cytotoxic T Cell
Killing of Target Cell by Cytotoxic T Cell
Once activated, a cytotoxic T cell recognizes a target cell infected with
intracellular pathogens through its TCR, which binds to MHC-I/epitope complexes
on the target cell. The cytotoxic T cell binds to the target cell and releases
perforin and granzyme proteins on to the surface
of the target cell. The perforin inserts into the plasma membrane of the target
cell and creates channels in the membrane. This allows extracellular fluid and
granulysin to flow into the cell causing bursting of the cell (cytolysis).
Additional granzyme molecules released by the cytotoxic T cell activate enzymes
in the target cell that cause fragmentation of its DNA and apoptosis. Cytotoxic
T cells also secrete gamma-interferon that attracts phagocytes to the site.
After detaching from the target cell, a cytotoxic T cell can continue to seek
and destroy other target cells. Memory cytotoxic T cells are inactive during
this immune response but can proliferate and differentiate into active cytotoxic
T cells if the infection returns.
 Activation and Dfferentiation of Cytotoxic T Cell
Activation and Dfferentiation of Cytotoxic T Cell
 Role of Helper T cell in Cellular Immunity
Role of Helper T cell in Cellular Immunity
 Killing of Target Cell by Cytotoxic T Cell
Killing of Target Cell by Cytotoxic T Cell
Antibody-Mediated (Humoral) Immune Response
Antibody mediated immunity works mainly against extracellular pathogens, which
include any bacteria, fungi or viruses that are in the extracellular fluids of
the body. Antibody-mediated immunity involves antibodies that bind to antigens
in body fluids. Cytotoxic T cells leave lymphatic tissues to seek out and
destroy a foreign antigen, however, B cells remain in lymphatic tissues. In the
presence of antigen, B cells in mucous associated lymph tissue (MALT), lymph
nodes or spleen become activated.
Although B cells can respond to an unprocessed antigen present in lymph or
intersitital fluid, their response is more intense if they process the antigen
and present it to helper T cells. Some antigens, such as the lipopolysaccharides
(LPS) in the cell wall of Gram-negative bacteria, can bind to BCRs as signal 1.
The second signal required for activation is provided directly by recognition of
a common microbial molecule or by extensive cross-linking of B-cell receptors by
repeating epitopes on the bacterial cell. Although this response is rapidly
produced, it is relatively weak compared to the response produced when B cells
are activated with assistance from helper T cells.
 Role of Helper T cell in Humoral Immunity
Role of Helper T cell in Humoral Immunity
The requirement for T-cell help means that before a B cell can be induced to make
antibody against the molecules of an infecting microbe, CD4+ T cells specific
for peptides from this microbe must be activated to produce helper T cells. This
occurs when naïve T cells interact with dendritic cells presenting these
peptides. During B cell activation, an antigen binds to the BCR. Most B cell
activation begins when a B cell takes in (by B-cell receptor-mediated
endocytosis) the same antigen that activated a helper T cell. The antigen is
processed, complexed with a class II MHC molecule and presented on the B-cell
surface. The TCR and CD4 of an activated helper T cell then binds to the
MHC-II/epitope complex. The B7/CD28 costimulation is not necessary because the
helper T cell was already activated. The helper T cell secretes interleukins,
especially IL-4, which stimulates the B cell to proliferate identical copies of
itself (clones) that differentiate into antibody producing plasma
cells and memory B cells. Memory B cells that encounter
the same antigen in the future results in a rapid proliferation and
differentiation of memory B cells into antibody-producing plasma cells and new
memory B cells.
All of the plasma cells of a particular clone are capable of secreting antibodies
specific for the antigen that originally stimulated the BCR of the B cell. IgM
is the first antibody secreted by activated B cells, but makes up less than 10%
of the immunoglobulin found in plasma; IgG is the most abundant. Much of the
antibody in plasma has therefore been produced by plasma cells derived from B
cells that have undergone isotype switching. Recall that the isotype refers to
the constant region of the antibody and determines the action that the antibody
takes to defend the body from this pathogen. So the early stages of the immune
response are dominated by IgM antibodies, later IgG and IgA predominate, with
IgE contributing a small but important part of the immune reponse.
While the underlying mechanism controlling what initiates an allergic response to
non-harmful agents is still largely unknown, research increasingly is
discovering how allergic responses proceed. It is known that mast cells,
eosinophils, and basophils are found in increased levels of activation in
allergic reactions. These cells produce histamine, bradykinin and other
chemicals that initiate a T cell mediated immune response. The CD4+helper T
subset of T cells are found in increased quantities in allergic reactions.
Knowledge of mediators of the allergic immune response will aid in developing
new therapies to treat or avoid allergic reactions.
The most significant difference between an innate and adaptive immune response is
immunological memory. This process only occurs with adaptive immunity. Innate responses
do not produce any memory cells. Therefore, each and every time an innate immune
response is initiated, new cells are recruited and the same degree of response occurs.
When an adaptive response is initiated and the T and B cells proliferate, specialized
memory cells are created along with the effector cells. Now, present in the tissues, are
many long-surviving cells able to recognize the antigen. If the antigen enters the body
a subsequent time, a rapid and more robust immune response occurs.
The first time the adaptive immune system encounters an antigen, the primary response
will take 5 –7 days before clonal expansion is complete and the lymphocytes have
differentiated into effector cells. During the initial primary exposure IgM is the most
prominent antibody secreted. The second and all subsequent times that the adaptive
immune system encounters this same antigen, the immune response will only take 1–3 days
to reach its maximal level and IgG is the prominent antibody secreted. Memory cells can
live for years and even decades. Therefore, as long as memory T or B cells are
circulating in the body, a faster, more robust immune response can be established. This
helps to eliminate the infection faster and more efficiently.
It is possible to acquire the immunological memory of adaptive immunity naturally or
artificially. Natural immunity results from natural exposure to an antigen. For example,
someone sneezes the influenza virus into the air and then it is breathed it into another
person’s nasal passageway. Although not always, but symptoms of the exposure usually
develop because the person is not immune during the first exposure. In artificial
immunity, an antigen is deliberately introduced into an individual to stimulate an
immune response. This introduced antigen is a vaccine and the process is vaccination.
Both of these immune responses are considered active processes and can persist for weeks
to a lifetime because memory cells have been produced. Passive immunity is not
long-lasting because the individual does not produce memory cells. Instead, this
temporary immunity results from the transfer of antibodies from a donor to a recipient.
An example of passive immunity results from a mother to her child as antibodies move
across the placenta before birth.
Example
Clinical Connection: Vaccines
Without immunological memory, vaccines would not work. Vaccines can prevent or
decrease the severity of infections. The mechanism underlying vaccination is that
exposure of the adaptive immune system to a small dose of an antigen will produce an
initial immune response. More importantly, this small dose of antigen establishes a
population of memory T and B cells that will live long-term in the body. When that
antigen is encountered again, the immune system is already primed. Upon a subsequent
exposure, a more robust and faster response is established and the pathogen is
destroyed faster. Thereby preventing clinical manifestations of the infection or
greatly reducing the clinical manifestations.
There are two types of vaccines commonly used: live-attenuated (viable) vaccines and
killed vaccines. Live-attenuated vaccines are living organisms that have been
modified in such a way that they will grow poorly in the human host and will
therefore produce immunity but not disease. People who receive the flu vaccine may
have mild flu-like symptoms for a few days but do not get the symptoms of disease
that may last for two weeks or more. Killed vaccines have been killed, usually by
irradiation, prior to administration. Both types of vaccination contain antigenic
proteins that will create memory cells to protect the person from future
infection.
Typically, use of live but weakened microorganisms in vaccines produces a more robust
immune response than does killed vaccines. Until now, the underlying reason why has
remained unknown. Recently, however, it was discovered that immune cells are able to
recognize whether a bacterium is live-attenuated or not (Sander et al, 2011). The
presence (and detection) of prokaryotic mRNA signals to the immune system that the
invading bacteria is live and, therefore, potentially infectious. As a result, a
strong immune response is initiated. It is not just presence of prokaryotic mRNA,
however, but specifically, the immune cells recognize a lack of the 3’-polyA tail on
the prokaryotic mRNA. This means that vaccines that are augmented with prokaryotic
mRNA might be able to produce an enhanced immune response in the future. This could
have a significant impact on vaccine design and development.
Example
Clinical Connection: Self-Recognition and Self-Tolerance
If cells are created to attack a foreign antigen, how does the immune system avoid
attacking itself? The immune system produces a powerful response to foreign
pathogens, but does not attack its own cells or molecules. To avoid attacking self,
several mechanisms have been established. The phagocytic cells can differentiate
between pathogenic organisms (bacteria, viruses, parasites and fungus) and human
cells. Pathogens express pathogen-specific signatures (PAMPs) that are not found on
mammalian cells. During development, B and T cells that recognize self-proteins are
eliminated during a process called clonal deletion. If a T cell does bind to its
antigen, but does not receive the proper costimulatory molecule stimulation, the T
cell is rendered inactive or tolerant. The T cell is anergic
and unable to properly initiate an immune response.
Both the innate and the adaptive immune responses have mechanisms in place to avoid
the destruction of their own tissues. Rarely, these mechanisms go awry and an immune
response to self-protein occurs. When they do malfunction, auto-immune diseases
arise. These are diseases where the immune system considers one’s own molecules as
foreign. Most of these diseases have a genetic component to them (they tend to run
in families), but also may require an environmental trigger.
Review of Adaptive Immunity
Unlike the innate immune response, the adaptive immune response responds to a
specific pathogen. During the adaptive response, B cell receptors recognize
pathogens free in body fluids. Additionally, these B cells and dendritic cells
engulf and digest the pathogen into peptide fragments. These peptides are bound
to a cellular protein called the major histocompatability complex (MHC) and
carried together to the surface of the phagocytic cell. This process is called
antigen presentation. These peptides presented in the context of (bound to) MHC
proteins are recognized by helper T cells. The helper T cell receptors bind to
the peptide and MHC protein and become activated releasing cytokines. Cytotoxic
T cell receptors recognize foreign peptides that were produced in the cell and
associated with MHC such as occurs in infected or tumor cells. Activated T
cells, called lymphoblasts, and activated B cells, called plasma cells, begin to
divide, creating hundreds of clones of the antigen-specific T or B cell. These
clones mount a cellular-mediated immune response or an antibody-mediated immune
response to rid the body of the pathogen. Memory T and B cells are also created,
which remain in the body and initiate an immune response if the antigen is
detected again. Vaccines are effective because of memory cells.

The proper functioning of body cells depends on precise regulation of the composition of
the interstitial fluid surrounding them. The composition of interstitial fluid changes
as substances move back and forth between it and blood and lymph. In addition, some
disruptions come from the external environment. Fortunately, the body has mechanisms to
try to inhibit foreign substances from entering the internal environment of the body and
if they do get in, immune responses help to remove or neutralize the invader before the
disruption leads to disease. Unfortunately, some factors or situations affecting the
lymphatic system and immune responses could disrupt homeostasis.
Inflammatory Response and Clinical Disruptions to Homeostasis
Lymph Nodes
Lymph nodes play key roles in the body’s immune function. Lymph nodes filter
harmful bacteria and other pathogens from the lymph, preventing them from
reaching the blood and circulating throughout the body.
Sometimes, however, the lymph nodes and appendix become overwhelmed by these
tasks, resulting in homeostatic imbalance. Lymph nodes can become swollen and
tender as the pathogen they are designed to fight infect them and result in
inflammation. This is a common symptom of many illnesses, and is often
(incorrectly) referred to as having “swollen glands.” Swollen lymph nodes often
occur behind the ears, on the neck, near the jaw or chin, and in the armpits.
Colds, the flu, tonsillitis, ear infections, and mononucleosis are common causes
of this swelling. Although pain and tenderness associated with swollen lymph
nodes typically diminishes within a few days of onset, the swelling can last for
several weeks after the onset of infection. After the infection has been
eradicated from the body, the lymph nodes return to their normal size.
Appendix
The lymph tissue of the appendix helps control microbes in the colon from moving
into the small intestine. Both of these structures activate the immune system to
mount an attack against these pathogens.
Acute inflammation of the appendix, termed appendicitis, can be preceded by a
number of possible causes. It is characterized by pain, high fever, and
increased white blood count. The infection results in edema and ischemia and my
progress to gangrene and perforation within 24 hours. Removal of the appendix
may be recommended.
Ruptured Spleen Can Create Homeostatic Imbalances
Homeostatic imbalances can also afflict the spleen, another lymph organ. Although
the ribs protect the spleen from trauma, direct blows to the left upper
abdominal area or even severe infection can result in rupture. When this
happens, the spleen spills blood into the peritoneal cavity. As a result, it has
been customary for such injuries to result in splenectomy, or total removal of
the spleen to prevent excessive blood loss or further infection. In recent
years, however, it has been found that the spleen has a significant capacity for
self-repair. As a result, splenectomies have become less common, as doctors
elect to leave part or all of damaged spleens in patients in the hopes the
tissue will repair itself. In severe cases, when the spleen has completely
ruptured and/or there is a high risk of infection, the spleen is still removed.
The liver and bone marrow take over many of the spleen’s functions, although
immune responses may be less robust in individuals without spleens.
Graft Versus Host Disease and Transplant Rejection
A bone marrow transplant is a procedure in which damaged or destroyed bone marrow
(the soft, flexible tissue inside bones) is replaced with healthy bone marrow
stem cells from a donor. Bone marrow is important because it is where blood
cells—including lymphocytes—are produced. When bone marrow stem cells becomes
damaged, as a result of certain types of cancer, chemotherapy, aplastic anemia,
or other diseases, a bone marrow transplant or stem cell transplant is needed so
that the body can continue to produce blood cells.
Graft versus host disease (GVHD) occurs when a patient receives a stem cell or
bone marrow transplant from someone who is not closely histocompatible. The
transplanted material attacks the recipient’s body. As mentioned previously,
bone marrow contains stem cells that produce white blood cells and other immune
system cells. No two people, except identical twins, have the same tissue type.
Therefore, each individual expresses unique proteins on their cells that are a
unique subset of major histocompatability complex (MHC) proteins. These unique
proteins help signal to the T and B cells that the cells are yours and should
not be attacked and destroyed. When a person receives a bone marrow transplant,
they are receiving some else’s immune cells. These cells may not recognize the
host cells as their own. As a result, the new immune cells can attack the host
cells, resulting in fever, weight loss, pain, rashes, and of primary concern,
the patient becomes more vulnerable to infection. Immunosuppressant medication
is typically given to patients in order to reduce the chances of GVHD
developing. If GVHD does occur, it is treated with high doses of
corticosteroids. GVHD occurs in about 30–40% of patients who receive donations
from relatives, and 60–80% of patients for whom the donor is not a relative.
Transplant rejection is a process in which a transplanted organ or tissue is
attacked by the recipient’s immune system. For example, a person receiving a new
kidney will require immunosuppressant drugs to prevent their immune cells from
attacking and destroying the new, foreign kidney. Transplant rejection can be
lessened by determining the MHC similarities between the donor tissue and the
transplant recipient and trying to match them as closely as possible. Rejection
is a cellular immune response via cytotoxic T cells inducing lysis of
transplanted cells as well as an antibody mediated response via activated B
cells secreting antibodies. The actions of the adaptive immune responses are
joined by components of innate immune responses via phagocytes and soluble
immune proteins.
Imbalances of Immune Function
Autoimmune Disorders
Finally, homeostatic imbalance can occur if the immune system fails to
distinguish self from non-self, resulting in attack on self-cells and organs by
auto-antibodies and self-reactive T cells. Autoimmune disease appears to be
caused by failure of the tolerance processes to protect the host from the action
of self-reactive lymphocytes. In an organ specific autoimmune disease, the
immune response is directed to an epitope unique to a single organ or gland.
Hashimoto’s thyroiditis, insulin-dependent diabetes mellitus (pancreas),
myasthenia gravis (acetycholine receptors on muscles) and Crohn’s disease (GI)
are examples of organ specific autoimmune diseases. Autoimmune diseases can also
be directed toward a broad range of epitopes and involve a number of organs and
tissues. Systemic lupus erythematosus, multiple sclerosis and rheumatoid
arthritis are examples of systemic autoimmune diseases. A variety of mechanisms
have been proposed for induction of autoimmunity and evidence exists for each of
these mechanisms. Indeed there may be different pathways leading to autoimmune
reactions. Current treatments include immunosuppressive drugs, thymectomy and
plasmapheresis for diseases involving immune complexes. Also, blockers of some
cytokines show success in several autoimmune diseases.
Systemic Lupus Erythematosus (SLE), or lupus, is an autoimmune
disease that results from an improper immune cell balance. It is a chronic
inflammatory immune disorder that results from an increase in a subset of helper
T cells, and an improper activation of B cells and macrophages. This results in
the activation of B cells that produce antibodies targeting the nucleic acids in
the cells of several organs in the body. Depending on the B cells that are
activated, many different locations can be affected including the brain,
digestive tract, heart, lung, skin, or kidneys. Arthritis in the joints is a
common symptom on patients with SLE.
SLE is more prevalent in women than in men. Symptoms can arise between the ages
10 to 50 and range in severity. Depending on the affected organ, symptoms can
include chest pain, headache, migraine, fever, fatigue, vomiting, arrhythmias,
malaise, hair loss, mouth sores, and the characteristic butterfly skin rash.
This rash occurs in about 50% of all cases. The cause (etiology) of SLE is still
unknown and an active area of research. There is no cure for SLE, but in most
patients the disease can be managed and outcome (prognosis) for patients has
been improving over the years.
Acquired Immunodeficiency Syndrome (AIDS) is caused by the
retrovirus, human immunodeficiency virus (HIV). HIV infects T cells,
specifically the T helper 2 subset (Th2) of T cells, in the human host. These
Th2 cells are also called CD4+ cells because of the presence of the CD4 protein
on its surface. Once inside the cell, HIV hijacks the host transcriptional
machinery to replicate the virus nucleic acids. After the nucleic acids have
been replicated the synthesis of virus proteins can occur. These newly
synthesized viruses exit the cell and infect another T cell. In the process, HIV
destroys the CD4+ cells in the body. By removing this population of immune
cells, the immune system of an infected person can no longer function properly.
Recall that the cytokines secreted by activated CD4+ helper T cells provide the
second signal for activation of cytotoxic T cells and also enhances activation
and proliferation of B cells and natural killer cells. Over time, as the CD4+ T
cell population drops, other viruses, bacteria, or fungi take advantage of the
weakened immune system and can gain access and proliferate in the host.
Therefore, people infected with HIV will commonly also be infected with herpes
simplex virus, pneumocystic pneumonia, tuberculosis, Candida, and more. Certain
cancers, including Karposi’s sarcoma and lymphomas involving the B cells of the
lymphatic system, are more prevalent in HIV infected patients. These
co-afflictions contribute to illness and further damages the immune system of
the patient. When the CD4 subset becomes very low, wasting syndrome, AIDS
dementia, and other diseases that destroy the organs develop. While there is no
cure for AIDS, medications to manage the disease exist. These long-term
medications have greatly decreased the short-term mortality rate by maintaining
CD4 cell counts, and patients with HIV are able to live much longer and more
productive life.
Lymphoma
There are many types of cancer that affect either T or B cells. These are
collectively called lymphomas. More than 30 types of
lymphomas have been detected. Some are aggressive and fast-growing lymphomas,
while others are less aggressive and slow growing. Lymphomas can arise at any
age and occur within the germinal centers of lymph organs and nodes. Chromosomal
changes can occur during any stage of development of the T or B cells. The most
common form of lymphoma is the diffuse large B cell lymphoma (DLBCL). There are
actually three subtypes of DLBCL, depending of the stage of development of
chromosomal changes. DLBCL is an aggressive, fast-growing lymphoma that occurs
in 30-40% of all lymphoma patients. In DLBCL, B cells continuously proliferate
and do not undergo apoptosis. Chemotherapy is available to treat this
disease.
Follicular lymphoma occurs in 20% of lymphoma patients. B cells in different
stages of development undergo chromosomal changes leading to their uncontrolled
growth. Unlike DLBCL, follicular lymphoma is a slow growing, non-aggressive
cancer. Patients can live for a few years to greater than 20 years with this
disease. Specific chromosomal changes occur in follicular lymphoma, and
therefore it is detectable by a molecular test.
Mantle cell lymphoma is the least common lymphoma, occurring in approximately 5%
of cases. Unlike the DLBCL and follicular lymphomas that arise from the cells in
the germinal center, mantle cell lymphomas arise from cells in the mantle region
of the node. Mantle cell lymphoma displays alterations in the cell cycle that
controls the progression of the cell through replication and division. Mantle
cell lymphoma is an aggressive disease. Patient survival generally is only 2–5
years.
Allergy
Allergy or hypersensitivity reaction is an immune reaction to agents that are not
normally harmful. These could be substances like strawberries, pollen, cat
dander, or cockroach casings. Immediate hypersensitivities are allergic
reactions that require prior exposure to the antigen. In this first exposure
helper T cells produce IL-4, which drives the plasma cells to produce antibodies
of the IgE isotype. The IgE binds to receptors present of mast cells for this
isotype. Once enough IgE antibodies are present on mast cells, exposure to the
same antigen induces mast cells to respond with an inflammatory response that
releases histamine, a chemical that increases the dilation of the lymph and
blood vessels. In addition, histamine produces watery eyes, itchy nose, and
cough. Delayed hypersensitivities take hours to days to develop and are caused
by T cells. The most common types result from contact of a substance with the
skin or mucous membranes. For example, poison ivy, soaps, and drugs can cause a
delayed hypersensitivity reaction. The substance is absorbed by the epithelial
cells, which are then destroyed by T cells. The inflammation and tissue
destruction causes intense itching.
Below is an activity that provides several sections of content and questions that will help
you recall, organize and apply what you have learned in this unit to a the condition of B-cell
Chronic Lymphocytic Leukemia. Explore the following activity to learn how this most common
form of Leukemia affects the body.
The activity consists of three sections.
- The top left panel contains an image with hotspots to be used for navigation.
- The top right panel contains the information you will need to solve the problems and questions that appear for each section.
- The bottom panel presents activities and questions unique to each section.
Be sure to click on each hotspot to ensure you complete all of the questions within this
activity. When you are sure you have finished each section, complete the follow-up activity
directly below this one.
B-cell Chronic Lymphocytic Leukemia
Introduction
Introduction
Most people who hear the word leukemia consider it an ominous word. It means “white
blood,” a term coined by one of the fathers of oncology, Rudolf Virchow, in 1847. Over
the past two centuries, research has shown cancer to have many types with many different
outcomes. Leukemia itself has many varieties depending on the cell type that
proliferates (divides) abnormally, from acute aggressive to chronic, slowly developing
types.
B-cell chronic lymphocytic leukemia is the most common form of leukemia.
It affects B cell lymphocytes, which are produced in the bone marrow and develop in
lymph nodes. Their primary function is to fight infections by producing a variety of
antibodies. In this form of leukemia, abnormal B cells begin to grow out of control and
accumulate - effectively crowding out healthy blood cells.
Consider the following scenario: A middle-aged man presents with complaints of
acute onset of abdominal discomfort and fullness, as well as weight loss. The patient
also complains of shortness of breath and fatigue. Observe the diagram below, showing
the results of a physical examination. Using the hotspots, explore the various symptoms
of his condition. After exploring each of these sections, complete the activity below
this lab.
Fatigue and Loss of Appetite
Fatigue is a complex symptom that can have a variety of causes, however it is mostly
associated to lack of energy. Take a moment to consider where energy comes from in our
bodies.
Lymph Node and Spleen Swelling
Lymphadenopathy and splenomegaly are the words for swelling of lymph nodes and spleen.
They both belong to the lymphatic system, although they differ in several aspects.
Shortness of Breath
The main function of the respiratory system is to obtain oxygen from the air and
eliminate carbon dioxide from the tissues. Oxygen crosses from the alveoli in the lungs
to the blood, where it is transported in hemoglobin.
Liver Enlargement
The liver is the body’s largest internal organ. It is also required for survival, due
to its many important functions.
 Enlarged (left) and normal (right) liver in the abdominal cavity. Observe how
the enlarged liver presses on the surrounding structures, including the stomach,
intestines, and gallbladder (in green).
Enlarged (left) and normal (right) liver in the abdominal cavity. Observe how
the enlarged liver presses on the surrounding structures, including the stomach,
intestines, and gallbladder (in green).
Repeated Infections
Leukemia patients have a tendency to suffer from repeated infections. During
infections, microorganisms such as bacteria, viruses, fungi, or parasites penetrate the
body’s defenses causing diseases. When healthy, the immune system is remarkably
efficient keeping invaders at bay.
Bone and/or Joint Tenderness/Pain
It is a common misconception to see bones as static and unresponsive. In fact, bones
are very dynamic organs, with a rich supply of blood and nerves through their
periosteum. Think about the pain of a damaged bone but also the speed of its recovery-
much faster than that of cartilage. Processes affecting the structure of bone and the
shape of joints tend to be painful.
Spotting, Easy Bleeding and Bruising
Bruises are normally caused by rupture of small blood vessels under the skin if we get
hit or bump into something. In the case of B-CLL patients, bruises appear without any
major trauma, in addition to easy bleeding of the mucosa. These symptoms point to
deficiencies in blood hemostasis
LAB TEST: Blood Cell Count
Here is a summary of some relevant blood tests for a B-CLL patient.
| TEST |
Normal |
B-CLL |
| White blood cell count ( per μl) |
4,500-11,000 |
Elevated |
| Lymphocyte count (per μl) |
1,200-4,000 |
>10,000/μl |
| Hemoglobin (g/dl) |
12-18 |
11< |
| Platelets (103 per μl) |
150-400 |
100< |
Blood Cell Morphology
All blood cells derive from hematopoietic stem cells in the bone marrow. In leukemias and
lymphomas, differentiation of cells stop at a certain stage, and immature cells divide
aggressively, “drowning out” other normal populations. One way to diagnose or further
explore leukemias is to observe the morphology of the cells in the blood or the bone
marrow. The slide below is a bone marrow aspirate of a B-CLL patient- you can observe an
elevated number of large lymphocytes with an immature appearance.
All of the systems of the human body functioning together comprise the total human
organism, the highest level of organization. All body systems influence and interact
with one another. They work together to maintain health and provide protection from
disease. The lymphatic system returns filtered fluids to blood preventing plasma loss
and interstitial edema. Water and solute must be correctly proportioned in the various
compartments of the body to maintain fluid balance. Fluid balance allows for healthy
circulation of substances throughout the body, optimal distances for diffusion of ions
and molecules, and proper ion concentration for excitable tissues. In addition, the
lymphatic system helps protect the whole organism from external invasion of foreign
substances into the body that may cause disease and also removes damaged or worn out
body cells as a normal component of tissue repair and maintenance.
The following table highlights some of the lymphatic system interaction with several
other body systems.
| Body System |
Lymphatic System Support |
Lymphatic System Benefits/Effects |
| Cardiovascular |
Returns filtered fluids and proteins to blood preventing interstitial edema and
loss of plasma volume. |
Provides transport for white blood cells from site of their origin to their
effector site. |
| Endocrine |
Thymus gland produces hormones that regulate T cell maturation |
Hormones coordinate leukopoesis; Cortisol is an immune suppressant |
| Muscular |
Fluid balance provides proper ion concentration and subsequent membrane
potentials for contraction |
Skeletal muscle pumps help lymph flow through lymphatic vessels |
| Nervous |
Source of microglial cells which remove debris from CNS. Cytokines promote
sleep; cytokines target temperature regulation setpoint in hypothalamus during
fever production |
Sleep optimizes immune response; hypothalamus resets body temperature during
fever production to enhance immune response; hypothalamus releases corticotropin
which activates the pathway for cortisol release which has anti-inflammatory
effects |
| Reproductive |
Antibodies cross placenta providing passive immunity to fetus and antibodies in
mother's milk provide passive immunity to newborn |
Immune system undergoes important modifications during pregnancy; critical that
mother tolerate fetus (foreign graft); sex hormones may be anti-inflammatory but
may exacerbte antibody mediated autoimmunity |
| Respiratory |
Prevents interstitial fluid accumulation so that distance for gas exchange is
optimal; MALT, alveolar macrophages inhibits pathogens that penetrate mucous and
alveolar membranes |
Gas exchanges in lungs provides oxygen to lymphatic cells and removes waste
carbon dioxide from these cells necessary for viability of cells |
| Skeletal |
Osteoclast, which helps regulate calcium balance, is derived from monocyte |
Red bone marrow is site of white blood cell formation |
| Integumentary |
Assists in interstitial fluid volume balance. Leukocytes remove pathogens and
help wound repair |
Anatomic and chemical barrier component of innate body defenses |
| Urinary |
Assists kidneys in fluid balance by helping maintain fluid in the vascular
compartments; MALT helps defend against pathogens |
Urine flow helps prevent pathogen entrance into body as part of innate
immunity |
| Digestive |
Lymphatic lacteals transport dietary lipids from small intestine to blood; GALT
inhibits pathogen that penetrate mucous membranes |
Provides nutrients for lymphoid organs; gastric acidity is component of innate
immunity |
In this unit, we’ll explore the structure and function of the nervous system. The nervous
system is often referred to as the master controller of the human body. Like the
endocrine system, the other internal control system of the human body, the nervous
system is specialized for communication of information from one part of the body to
another. The nervous system communicates quickly using neurons, the specialized cells of
the nervous system. Neurons can convey and process information using electrical and
chemical signals. Ultimately, neural communication helps coordinate body activities and
ensures we maintain homeostasis.
Imagine that you decide to bake a cake for your Anatomy and Physiology professor’s
birthday. You find a recipe, locate the ingredients and then one by one, measure and add
each to the mixing bowl. You stir the mixture and before you know it, the cake is in the
oven and you can return to your homework. You start to salivate as a yummy chocolate
aroma wafts through the room. The timer goes off, but unfortunately, when removing the
pan from the oven you burn your hand through a hole in the hot pad. Rather than dropping
the cake, you make a split second decision to hold on, enduring the pain so that you can
set the cake pan down safely even though the consequences of a burnt hand will be with
you for a few days. All aspects of baking a cake from your decision to bake a cake, to
reading the recipe, measuring and mixing ingredients, smelling a delicious chocolate
aroma and automatically starting to salivate in case you decide to sneak a piece of
cake, and withdrawing your hand to prevent a burn are functions of the nervous system.
Although quite different behaviors, they all include the general functions of the
nervous system listed below:
-
The nervous system detects changes in our internal and external environment (stimuli)
using specific neurons or specialized cells communicating with neurons called sensory receptors.
Sensory receptors can detect a variety
of different external and internal stimuli such as: skin temperature, light (for
vision), sound, chemicals in food (taste) and air (smells), pressure, pain, blood pH,
core body temperature, bladder distension as well as many other stimuli.
-
Sensory receptors transform stimuli into electric signals that
our nervous system can understand.
The nervous system cannot directly interpret
stimuli like light, heat or sound. The information has to be transformed into an
electrical signal for the nervous system to receive and process the information.
-
Sensory neurons transmit the electrical signals from the periphery
to the central nervous system (brain and spinal cord).
This information travels
from the sensory receptor (the site of transformation) along neural processes called
axons towards the central nervous system (CNS).
-
The central nervous system (brain and spinal cord) processes
incoming sensory information to generate “appropriate” responses and also to give us
the perception of the stimulus.
The signal can be compared to a normal value
(set point) or related to past experiences to determine if the stimulus requires a
response. Processing of the signal is called integration. In order to perceive a
stimulus, the sensory information must be transmitted to specific areas of the
brain.
-
The central nervous system sends commands (electrical signals
passed along neurons) out to the target tissues to produce the response.
The
target tissues of the nervous system are muscles and glands.
The large brain of humans is perhaps the most important evolutionary advance for the
species. At the minimum, it is the characteristic most of us consider the distinguishing
characteristic of a human. This module outlines the structural and functional
relationships of the human brain.
 Superior view of the brain. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Superior view of the brain. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
 Lateral view of the brain. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Lateral view of the brain. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The dominant portion of the human brain is the cerebrum. It is the large upper part of
the brain, distinguished by the gyri (folds) and sulci (folds)
of the surface. The cerebrum is clearly split into left and right
hemispheres; the split is the deep longitudinal fissure.
The cerebrum sits atop and around the midbrain, which leads into the
brainstem. Situated essentially behind the midbrain and under the
cerebrum is the distinctive cerebellum.
The inside of the brain is characterized by regions of gray matter and
white matter. The gray matter is mostly cell bodies, dendrites, and
synapses and forms a cortex over the cerebrum and cerebellum, and also forms some nuclei
deeper in the cerebrum. White matter is myelinated axons forming tracts. (These definitions
and components of gray and white matter are similar to the ones for the spinal cord, although
their arrangement will be different as you will discover later in this unit.)
The cerebral white matter tracts are classified as
- Projection tracts-from higher to lower, from cerebrum to brainstem and spinal
cord
- Commissural- across hemispheres
- Association- within same hemisphere
The gray matter of the cerebral cortex includes:
- Stellate cells- receive sensory input and process information locally
- Pyramidal cells- extend to other parts of the CNS
- Neocortex- 6 layered tissue of recent evolutionary origin
Like the spinal cord, the brain is covered and partially protected by connective tissue
meninges. From outermost (bordering the skull bones) to the
innermost (adjacent to the nervous tissue) they are the dura
mater, arachnoid mater, and pia mater.
The dura mater folds into two layers, a periosteal layer fused to the skull bones, and a
meningeal layer. In some areas, these layers are separated by a dural sinus, a space
used to collect blood. Some areas may also contain a subarachnoid space
or a subdural space.
 Meninges.
Meninges.
Cerebrospinal fluid (CSF) is a clear, colorless liquid that bathes
the external surfaces of the brain. It is constantly produced, flows through the network
of ventricles, and is reabsorbed. CSF functions in cushioning and supporting
the brain by buoyance, and in chemical stability of the brain, by transporting nutrients
and wastes respectively.
The ependymal cells lining the ventricles produce the CSF. Then the CSF flows throughout
the brain in the ventricles. Each cerebral hemisphere contains a lateral ventricle. Each lateral ventricle drains through an interventricular foramen into the third ventricle. The
third ventricle sits in the midbrain region. The CSF then flows into the cerebral aqueduct to the fourth ventricle.
Before being reabsorbed, the CSF enters one of two lateral apertures
or a median aperture, and then fills the subarachnoid space.
Reabsorption of CSF occurs there by the arachoid villi and enters
the venous blood.
 CSF flow through the ventricles.
CSF flow through the ventricles.
The choriod plexus is the network of blood vessels and ependymal
cells on surface of the ventricles. The ependymal cells of this choroid plexus secrete
the CSF. Overall the close proximity of ependymal cells and blood vessels create a blood-brain barrier (BBB). The brain
requires large amounts of oxygen and glucose but other items in blood may harm it, hence
the barrier. At the capillaries there is a BBB of tight endothelial cells and basement
membrane; at the choriod plexus the blood-CSF barrier due to tight junctions between ependymal
cells
Next, the main structural areas of the brain will be surveyed with some of their major
functions. We will go in general order of most primitive brain region to most
evolutionary advanced region, or, in other words, from basic functions to more advanced
functions.
The most inferior part of the brain, the medulla oblongata, appears as a
thickening of the spinal cord. Many of the cranial nerves originate here (see below).
The medulla oblongata contains nuclei that control many basic functions, including the
cardiac center, the vasomotor center, the respiratory centers, and many other
involuntary functions such as swallowing, coughing, salivating, sweating, and
gastrointestinal secretion.
 Posterolateral view of the brainstem.
Posterolateral view of the brainstem.
In humans, the pons is the next most superior feature of the brain; the pons
looks like a forward-facing bulge in the brainstem above the medulla oblongata. The pons
relays signals between cerebrum and cerebellum, including sleep, hearing, taste, and
posture to name a few.
The cerebellum is a smaller, highly folded structure in the back of the
brain, behind the pons. Like the cerebrum, it is split into hemispheres, with a
flattened area down the center called the vermis. The folds are folia
and grooves are sulci. The white matter forms a distinctive
arbor vitae ("tree of life"). The cerebellum is concerned with muscular coordination,
special perception, and tactile perception, and some planning and scheduling tasks.
The midbrain is a small region of gray matter nuclei involved in different
motor and sensory functions and connecting white matter pathways. These structures
include:
- Cerebral peduncles- anchor cerebrum to brainstem
- Tegmentum- to/from cerebellum for motor control
- Substantia nigra- inhibitory relay (the area destroyed in Parkinson disease)
- Central gray matter- pain awareness
- Tectum- include the inferior and superior colliculi for hearing and
vision
- Red nucleus- subconscious motor commands and muscle tone
The reticular formation is a series of gray matter extensions from the
midbrain through the cerebellum. They are involved in
- Somatic motor control, including the pattern generators
- Cardiovascular control
- Pain modulation
- Sleep and consciousness, including habituation
The forebrain is the large overarching region of the brain. In the center,
above the midbrain are the thalamus and hypothalamus.
 Nuclei of the thalamus.
Nuclei of the thalamus.
The thalamus is a set of nuclei mainly involved in the relay of sensory signals. Those
will be covered in more depth in sensory units. Other thalamic nuclei are involved in
memory and emotions.
The hypothalamus is a set of nuclei situated underneath the thalamus. The main function
of the hypothalamus is control of the endocrine system and as such will be covered in
more detail there. Other nuclei of the hypothalamus are involved in many autonomic
functions such as thermoregulation, food and water intake, biological cycles, and
emotions.
The cerebrum is the major anatomic feature of the human brain. The cerebrum
is made of lobes. The frontal lobe is from the frontal bone to central
sulcus and is involved in voluntary motor functions, planning and foresight, memory,
mood, emotion, social judgment, and aggression.
The parietal lobe is the upper part of brain in each hemisphere from the
central sulcus to parietal-occipital sulcus; this lobe is primarily involved in sensory
reception and integration.
The temporal lobe of each hemisphere sits under the parietal lobe and the
lateral sulcus; this lobe has roles in hearing, smell, learning, memory, visual
recognition and emotional behavior.
The lobe furthest to the rear of the head is the occipital lobe, and it
contains the visual center.
The insula is a mass of cortex underneath the outer lobes, found beneath the
frontal and temporal lobes.
 Locations of the basal nuclei.
Locations of the basal nuclei.
The basal nuclei are clusters of cell bodies found at the bottom (base) of the cerebrum, surrounding the thalamus.
They include the caudate nucleus, and the putamen, and globus pallidus, (last two collectively known as the lentiform nucleus
and all 3 are sometimes referred to as the corpus striatum) and are involved in motor control.
The limbic system is a loop of cortical structures in temporal lobe surrounding corpus
callosum and thalamus. These structures include the hippocampus, amygdala, fornix, and
cingulate gyrus. More on the hippocampus and amygdala later in the presentation of
higher level functions.
Higher brain functions are ones generally assigned to regions in the forebrain.
Most of these locations were discovered by studying people who had lesions in
these regions, and as a result, were defective in one of these functions.
For most functions, there is a localization between the left and right hemispheres,
called cerebral lateralization. The left hemisphere generally is stronger
in motor, mathematical, and language skills, while the right hemisphere generally
emphasizes spatial and tactile skills. The two hemispheres are connected by the
corpus callosum.
Cognition is awareness perception, thinking, knowledge, and memory. Its most
basic definition is the integration of sensory and motor systems. The cerebral lobes
contain most of the regions associated with cognition. An important memory forming
center is the hippocampus.
Emotion is deeper feeling, resulting from memory and learned behavior. Many
emotions are rooted in the hypothalamus and amygdala.
 Location of the motor (precentral gyrus) and sensory (postcentral gyrus).
Location of the motor (precentral gyrus) and sensory (postcentral gyrus).
Sensation is the perception of one of the senses. Here, we will simply point
out the importance of the post-central gyrus for the interpretation of
general senses. General senses are ones from widely distributed
receptors, such as touch, pressure, temperature, pain. These pathways end at the
post-central gyrus, or sensory cortex. The cortex exhibits somatotophy:
point by point correspondence of body locations to brain locations. The point by point
correspondence gives more area of the cortex to regions that are well innervated with
sensory receptors, such as fingers, and face; meanwhile regions that do not have large
sensory innervation have correspondingly smaller areas on the sensory cortex.
The special
senses are interpreted in their own specialized cortical regions,
and are discussed in another section of this course.
Motor control refers to the initiation and proper coordination of the movement of a
muscle. For a skeletal muscle, the intent to contract a skeletal muscle begins in motor
association area (frontal lobe). The signal is then sent to the precental
gyrus or primary motor area, which is the origin of the upper
motor neuron. The precentral gyrus also exhibits somatotophy. Body areas, such as lips
and fingers, which have fine motor control, have a large area dedicated on the primary
motor cortex while areas that do not have fine control have a correspondingly smaller
area.
Language includes abilities such as reading, writing, speaking, and comprehending words.
At least two major areas are involved in the recognition and formation of language.
Wernicke’s area, within the parietal and temporal lobes, is involved in
the recognition of language. Broca’s area is involved in the formation of
words.
The Cranial Nerves provide input to and output from the brain. The numbers, names, and a
short functional description are below.
 Orientation of the cranial nerves.
Orientation of the cranial nerves.
- Olfactory: sensory for smell
- Optic: sensory, process visual information
- Oculomotor: motor, movement of eyes and smooth muscles controlling pupil and
lens
- Trochlear: motor, eye movements
- Trigeminal: sensory of upper, and mid face and upper jaw; motor for muscles of
chewing
- Abducens: motor, eye movements
- Facial: motor for facial expression, tears and salivary glands; sensory for
taste
- Vestibulocochlear: sensory, hearing and equilibrium
- Glossopharyngeal: motor for mouth (swallowing) and for regulation of blood pressure;
sensory for tongue and pharynx and outer ear
- Vagus: motor for swallowing, speech, cardivascular and digestive regulation; hunger
and fullness; sensory from visceral organs and taste. Main parasympathetic
nerve
- Accessory: swallowing, and head, neck, shoulder movement
- Hypoglossal: tongue movements
The nervous system is critical to many of our homeostatic feedback loops. In most of
these loops, the structures of the nervous system make up more than one component, and
carry out more than one function in these loops. For example, specialized nerve endings
often act as sensors (receptors), information is carried along nerves and/or tracts of
the spinal cord, integration occurs within the CNS, and spinal cord tracts and nerves
carry the responding information back out to the effectors. The spinal cord is a nervous
system structure dedicated to relaying information from the periphery to the brain and
back, as well as carrying out certain levels of integration, such as those found in many
reflexes. The structure of the spinal cord aids it in carrying out these relaying and
integrative functions.
The spinal cord is a central nervous system structure that extends inferiorly from the
brain stem and into the lower back. Throughout its length, it is enclosed within the
spinal column, with the cord passing through the vertebral foramen of the vertebrae. In
an adult, the spinal cord itself terminates at a point called the medullary cone, at approximately the level of the first lumbar vertebrae (L1). Below the
medullary cone, the vertebral canal contains a bundle of nerve roots called the cauda
equina.
 Spinal cord.
Spinal cord.
This figure shows other important features of the spinal cord, many of them related to the
spinal cord's function of relaying information. Starting between the base of the skull
and the first cervical vertebrae, and continuing into the sacral
region of the spinal column, a pair of spinal nerves extend from the spinal
cord (although information is transmitted in both directions on sensory and motor
neurons within these mixed nerves). All but the first spinal nerve (C1)
pass through the intervertebral foramen of the spinal cord, whereas spinal
nerve C1 passes between the occipital bone and vertebrae C1. In all there are 31 pairs of
spinal nerves that carry information to and from the spinal cord and the periphery of
the body. Note that not all of the spinal nerves arise from the cord at the level of the
vertebrae between which they pass. This is most obvious when considering those spinal
nerves arising in the lower lumbar and sacral regions. The nerve roots for these nerves
arise from the spinal cord at, or near, the medullary cone, which you will recall is
near the L1 vertebrae. These roots are contained within the cauda equina until passing
out of the spinal column. Because the spinal nerve roots don’t always originate at the
level of the vertebrae that they pass through, the segments of the spinal cord are named
for the spinal nerve to which they give rise. For example, segment S2 of the spinal cord
would be located near the T12 vertebrae.
Because the spinal cord terminates near vertebrae L1, and there is a lot of body tissue that
needs to be innervated below this level, there are a significant number of nerves
arising from the lower aspect of the spinal cord. This leads to an area of increased
spinal cord thickness in the lumbosacral regions of the spinal cord
(corresponding to a region associated with the inferior thoracic vertebrae) called the
lumbar enlargement. There is a corresponding cervical
enlargement in the cervical segments that give rise to nerves innervating the
upper limbs.
Recall that the central nervous system tissues can generally be divided into white matter
and gray matter. White matter is the myelin-containing region composed of axons, which
make up the tracts of the CNS. These carry information between different regions and
structures in the CNS. Gray matter contains the cell bodies and dendrites and therefore
is the site of synaptic transmission.
In the cortex of the brain, gray matter makes up the cortical
(outer) regions, while
the white matter tracts tend to make up the majority of the deep
tissues of the brain,
although there are exceptions to the latter, such as the deep basal
and thalamic nuclei that are composed of gray matter. In contrast to
this general arrangement of the brain, the spinal
cord is arranged with the white matter surrounding the central gray
matter, indicating that the spinal tracts carry information up and
down the cord along
the outer aspects, while synaptic transmission tends to occur more
centrally.
 Cross-sectional view of a spinal cord segment.
Cross-sectional view of a spinal cord segment.
In the image above, you can see how the
central gray matter is somewhat butterfly shaped, with each side of the “butterfly”
containing a posterior (dorsal) horn and an anterior (ventral) horn. Each of the horns
is contiguous with the posterior and anterior spinal nerve roots, respectively. The
posterior root of the nerve carries sensory information into the posterior horn, often
synapsing there. The anterior horn contains the cell bodies of somatic motor neurons,
and it sends its axons out the anterior root of the spinal nerve to the muscle cells it
innervates. The lateral horn is not found at all levels of the spinal cord, but is
limited to thoracic and lumber segments of the cord. This is because the lateral horns
contain the neurons of the sympathetic nervous system, which leave the cord only in
these segments. Even though the cell bodies are found in the lateral horns, their axons
leave via the anterior nerve roots, just like those that control skeletal muscle. The
matched horns on each side of the “butterfly” are connected via the gray commissure, which also surrounds the cerebrospinal fluid filled central canal.
The white matter of the spinal cord is divided into columns. Each
segment of the cord contains matched posterior, lateral and anterior columns. The
anterior columns and posterior columns are partially separated by the anterior median
fissure and posterior median sulcus, respectively. Each pair is also connected by a
commissure of white matter that runs adjacent to the gray commissure, termed the
anterior and posterior commissures. The columns are further divided into tracts that
carry sensory information up the spinal cord (ascending tracts) and motor information
down the spinal cord (descending tracts).
 Cross-sectional view of the length of the spinal cord.
Cross-sectional view of the length of the spinal cord.
Although each segment of the spinal cord has similar features,
there are some
differences along its length, as you may be able to determine from
the image above. The main difference is that
the ratio of gray matter to white matter varies among segments of the
spinal cord. At
the lower levels of the spinal cord there is a greater ratio of grey
matter to white
matter. This should make sense, as there are less ascending and
descending tracts of
whiter matter as you move lower. As previously mentioned, the lateral
horns are only
found in the thoracic and lumber regions of the spinal cord, where
they contain the motor
nuclei of the sympathetic nervous system. Finally, the size of the
anterior and
posterior horns varies, depending on the amount of tissue they are
innervating. For example, the thoracic segments have relatively
small anterior horns, as there is little skeletal muscle to innervate
in the thorax and
abdomen, while the cervical and thoracolumbar regions have large
anterior horns, used to
innervate the skeletal muscles of the arms and legs, respectively.
The white matter of the spinal cord is divided into the paired
posterior (dorsal), lateral, and anterior (ventral) columns. These columns are sometimes
called funiculi (or funiculus when singular) and are made up of axons that are traveling
up (ascending) or down (descending) the spinal cord. The ascending tracts generally
carry sensory information from the periphery to the brain, while the descending tracts
carry motor signals to muscles and glands.
The columns can be further divided into tracts (sometimes called fasciculi), which is a
way of functionally grouping the neurons based on similar origin, destination and
function. These tracts are often named for the structures that they connect. For
example, the spinothalamic tract indicates that the fibers are carrying information from
the spinal cord to the thalamus of the brainstem. You may note from its name that it is
an ascending tract, so the information that it carries is sensory.
Some of the tracts cross over (decussate) either in the spinal cord or brainstem, and
when this occurs, the relationship between the origin and destination is termed
contralateral. Much of our motor control is contralateral. For example, your right arm
is mainly controlled by the motor area in your left brain. When the origin and
destination of a tract are on the same side of the body, it is referred to as an
ispsilateral relationship.
 Cross-section of the spinal cord, indicating how the white matter
columns can be divided into various tracts.
Cross-section of the spinal cord, indicating how the white matter
columns can be divided into various tracts.
| Spinal Tracts |
|---|
| This table lists the major spinal tracts, indicates if they
decussate, and provides a brief description of the types of information that they
carry. |
| Tract |
Column Location |
Dessusitation location |
Function |
| Ascending Tracts - Sensory |
| Gracile fasciculus |
posterior |
medulla |
below level T6: limb and trunk position sensations; deep touch; visceral pain; vibration |
| Cuneate fasciculus |
posterior |
medulla |
level T6 and above: limb and trunk position sensations; deep touch; visceral pain; vibration |
| Spinothalamic |
lateral and anterior |
spinal cord |
light touch, tickle, itch, temperature, pain, and pressure sensations |
| Spinoreticular |
lateral and anterior |
some fibers of the spinal cord |
pain sensation from tissue injury |
| Posterior spinocerebellar |
lateral |
none |
proprioception - feedback from muscles |
| Anterior spinocerebellar |
lateral |
spinal cord |
proprioception - feedback from muscles |
| Descending Tracts - Motor |
| Lateral corticospinal |
lateral |
medulla |
fine limb control |
| Anterior corticospinal |
anterior |
spinal cord |
fine limb control |
| Tectospinal |
anterior |
midbrain |
head-turning reflex in response to visual and auditory stimuli |
| Lateral reticulospinal |
lateral |
none |
posture and balance; awareness of pain regulation |
| Medial reticulospinal |
anterior |
none |
posture and balance; awareness of pain regulation |
| Lateral vestibulospinal |
anterior |
none |
posture and balance |
| Medial vestibulospinal |
anterior |
some fibers of the medulla |
head position control |
|
The spinal cord acts as a conduit for information traveling up and down its length. But
because most of this information has to either exit the spinal cord to send signals to
peripheral tissues (efferent transmission), or information from peripheral tissues needs
to be carried into the spinal cord (afferent transmission), there must be appropriate
structures for these types of transmission to occur. It is the 31 pairs of spinal nerves
and their related structures that provide the pathways for this interaction.
Sensory and motor fibers enter and exit the cord via rootlets that arise from both the
posterior and anterior aspects of the cord. Anterior rootlets carry motor
information out of the spinal cord (i.e. they contain efferent fibers) while the
posterior rootlets carry sensory information into the spinal cord (i.e. they contain
afferent fibers). Several posterior rootlets merge together to form the posterior root,
while several anterior rootlets similarly converge to form the anterior root.
 Rootlets and the passing of information to and from the spinal cord.
Rootlets and the passing of information to and from the spinal cord.
Along the posterior root is a ganglion,
where cell bodies of many of the
sensory neurons are found. These are unipolar neurons, such that
their dendrites extend
out to the peripheral tissues, and their axons project into the
dorsal horn of the
spinal cord, where they synapse. These unipolar peripheral neurons
are considered first
order neurons in the sensory pathway, while the neurons they synapse
with in the
posterior horn are considered the second order neurons of the sensory
pathway. It is the axons of these second order neurons that make up the
various ascending white matter tracts.
The anterior root does not contain a ganglion. This is because motor control is typically
a two neuron pathway. It starts with an upper motor neuron, whose cell body is in the
cerebral cortex or gray matter of the brainstem. This neuron projects its axon via a descending white
matter tract to a point in the spinal cord where it synapses in the ventral horn with a
lower motor neuron. The cell body of the lower motor neuron is in the gray matter of the
spinal cord, and it projects its axon out one of the anterior rootlets and through the
anterior root. Ganglia are only found where neuron cell bodies are outside the CNS.
Distal to the posterior root ganglion, the fibers of the anterior and posterior root
merge together and pass through the dura to become the spinal nerve. Because the spinal
nerves contain both sensory and motor fibers, they are considered a mixed nerve, as
opposed to either a sensory or motor nerve.
Just distal to the intervertebral foramen of the spinal column, the spinal nerve branches
into rami (singular: ramus).
In general, the posterior ramus communicates
with structures posterior to the cord, while the anterior ramus
communicates with
structures anterior to the cord. In spinal nerves T1-L2, the anterior
ramus gives rise
to a communicating ramus that communicates with the sympathetic
ganglia in the region. The sympathetic motor pathway involves two motor
neurons, so this ganglion houses the second motor neuron’s cell body.
In various regions of the body, the anterior rami from several spinal
nerves join
together and then branch again, in a complex network of nerves called
a plexus.
A plexus is a network of anterior rami from neighboring spinal nerves that come together
in a weblike or tangled network adjacent to the spinal cord, and from which new nerves
arise. These nerves contain fibers from several spinal nerves. The four main plexuses
are the cervical, brachial, lumbar and sacral. Some people consider the coccygeal plexus
a fifth plexus, although it is much smaller than the others.
The plexuses are complex networks, with four or more spinal nerve
rami contributing to
each of the four main plexuses, and with several nerves arising from
each. The following table lists the four main plexuses, the spinal
nerves that contribute to each,
and some of the main nerves that arise from them.
| Plexus |
Spinal Nerves that Contribute |
Example Nerves Arising From |
| Cervical |
C1-C5 |
Lesser Occipital |
|
Great Auricular |
|
Supraclavicular |
|
Phrenic |
| Brachial |
C5-C8, T1 |
Axillary |
|
Radial |
|
Musculocutaneous |
|
Median |
|
Ulnar |
| Lumbar |
T1-T4 |
Femoral |
|
Saphenous |
|
Obturator |
| Sacral |
L4-L5, S1-S4 |
Superior gluteal |
|
Inferior gluteal |
|
Tibial |
Before taking the quiz below, consider again the learning objectives for this unit. Could
you demonstrate each of these objectives? If so, you will be ready for the assessment
below. If not, consider reviewing content related to these objectives before attempting
the assessment.
- Describe the basic (overall) structure of the human brain.
- Explain the roles of CSF, ventricles, and the blood brain barrier.
- Correlate hindbrain and midbrain regions to their major function(s).
- Correlate forebrain regions to their major functions(s).
- Identify the location of major brain regions.
- Assign function(s) to each of the cranial nerves.
- Describe the gross anatomy of the spinal cord and spinal nerves and
specify their location relative to the anatomy of the vertebral column.
- Contrast the relative position of gray matter and white matter in
the spinal cord with the corresponding arrangement of gray and white
matter in the brain.
- Compare and contrast the anatomical features of the spinal cord in the cervical, thoracic and lumbar regions.
- Identify how spinal structures relate to each other: tract, root, ganglion, nerve, ramus, plexus.
The nervous system consists of two major divisions:
- The central nervous system (CNS) consists of the brain and the spinal cord, which
are enclosed in the skull and vertebral column, respectively.
- The peripheral nervous system (PNS) consists of all the neural tissue outside of the
brain and spinal cord. The PNS includes the cranial nerves and spinal nerves,
sensory receptors and ganglia (cell bodies (somas) of neurons that lie outside the
CNS). The nerves connect all other parts of the body with the CNS.
 Divisions of the Nervous System. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Divisions of the Nervous System. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The peripheral nervous system has several subdivisions. It is first divided based on
function into sensory (afferent) and motor (efferent) divisions. Each of these is
further subdivided into somatic and autonomic (visceral) divisions.
 Subdivisions of the Nervous System.This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Subdivisions of the Nervous System.This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The nerves that comprise the peripheral nervous system can be divided into two divisions
based on whether information is traveling into the CNS or information is leaving the
CNS. The sensory division of the PNS contains nerves carrying sensory
information into the CNS. These sensory nerves are also called afferents
(carrying toward). The motor division contains nerves carrying information
out of the CNS to target organs. These motor nerves are also called
efferents (carrying away).
The sensory (afferent) division of the PNS has two subdivisions. The
somatic sensory division conducts signals from receptors located in the
skeletal muscles and skin. The visceral or autonomic sensory
division conducts signals predominantly from organs contained in the thoracic
and abdominopelvic cavities (ex. heart, lungs, intestines, bladder, etc.).
The motor (efferent) division of the PNS is also subdivided into somatic and
visceral divisions. The somatic motor division controls the voluntary
actions of the skeletal muscles in the body. The visceral motor division,
more commonly called the autonomic nervous system, controls the actions of
cardiac muscle, smooth muscle, and glands. The responses in these targets are usually
involuntary.
The autonomic nervous system (ANS) is further subdivided into the sympathetic
division and the parasympathetic division. Generally, the
sympathetic division is involved in getting the body ready to respond to a physical
challenge or an emotional threat, classified historically as the “fight or flight”
division of the ANS. The parasympathetic division functions in opposition to the
sympathetic nervous system. It is responsible for "rest and digest" activities,
and is involved in salivation, digestion, urination and defecation.
Neurons are considered the simplest functional unit of nervous tissue. They are
long-lived (most live for your entire life), electrically active cells that consume a
lot of energy. Neurons are capable of responding to stimulation, conducting electrical
signals, and secreting chemicals that allow them to communicate with other cells. They
cannot usually regenerate if damaged since most neurons do not retain the ability to
divide. Neurons have anatomically and functionally distinct regions for receiving,
integrating and sending information from one part of the body to another.
 Like epithelia, neurons are polarized with
anatomically and chemically distinct regions. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Like epithelia, neurons are polarized with
anatomically and chemically distinct regions. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
A typical neuron, like that shown above, has two distinct processes or cytoplasmic
extensions on either side of a soma (cell body). On one side of the soma are short,
tapering processes called dendrites (Greek, dendreon – tree). Most
neurons have many, highly branched dendrites, although they may have as few as one.
Dendrites receive information from other neurons and transfer it to the cell body. The
greater the number of dendrites, the more information the neuron can collect to use
during decision making.
The soma (cell body) is the region of the neuron that integrates
all the incoming information from the dendrites. The cell body is somewhat spherical in
shape and for humans, typically ranges in size from 5 – 100 microns in diameter. The
soma contains the neuron’s nucleus and housekeeping organelles (e.g. mitochondria,
lysosomes , Golgi complex, rough endoplasmic reticulum, etc.). The soma is the only site
in a neuron that can synthesize proteins, neurotransmitters, or materials needed for
cell maintenance and repair. The soma is similar to the dendrites in that it can also
receive inputs from other neurons. The incoming information is coded as electrical
signals that are integrated to determine what, if any, response is required. If the
incoming signal is large enough, it travels to the process on the other side of the soma
called the axon. If the incoming signal is small, it dies out
before it reaches the axon.
The axon functions like a cable, relaying electrical signals away
from the cell body towards other neurons or cells (e.g. muscles,
glands).
Axons are also called nerve fibers. The axon
has three regions. As it emerges from the cell body, the axon forms a
structure called
the axon hillock, a tapered region that contains the initial segment, or trigger zone, where
propagating electrical signals called action potentials are initiated or generated. The
next part of the axon is the longest, typically a single, thin (.5 - 3 microns), almost
constant diameter process that extends to a target. Axons can be long, short or in
between.
Example
For example, neurons that command the big toe muscles to contract have dendrites and cell
bodies in the spinal cord and axons that leave the vertebral column to travel the length
of the leg to the big toe. For a 7 foot tall basketball player, the axon that controls
the big toe might be 3 feet long. On the other hand, short axons (a few microns long)
are found on interneurons, neurons that have all processes within the CNS and nearby
targets.
The axon may travel to its target as a single fiber, but some axons form branches called
collaterals, so that they can interact with not just one, but many target cells. The
third region of the axon is found when it reaches its target. Here the axon branches
extensively forming the synaptic terminals (terminal arborization). Each branch ends with a small swelling called a synaptic knob, which contains vesicles filled with chemical
messengers (neurotransmitters) that conduct the signal to the next
cell.
Neurons are classified according to 2 different characteristics: their morphology
(anatomical or structural features) and their function. Although we describe both here,
the functional classification will be used predominantly in later units.
 Neurons can be classified according to their
function. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Neurons can be classified according to their
function. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Functional classification of neurons is based on the direction of information flow along
axons relative to the CNS. Based on this criterion, there are 3 types of neurons:
sensory neurons, interneurons, and motor neurons.
Sensory (afferent) neurons are specialized for detection of sensory
information (e.g. light, pressure, vibration, temperature, chemicals, etc.). They
transduce physical and chemical stimuli into electrical signals and transfer this
information from the periphery towards the central nervous system for processing. In
many cases, sensory neurons have their dendrites, soma and a part of their axon residing
outside the CNS with axon terminals forming connections (synapses) with other neurons
within the CNS.
Interneurons (association neurons) are located entirely within the
central nervous system (with the dendrites, soma and axons of the cell all residing
within the CNS). Interneurons are also referred to as association neurons, in part
because they are sandwiched between sensory and motor neurons where they integrate and
distribute sensory information and coordinate motor output. Interneurons account for 90%
of all neurons of the CNS and therefore are the most numerous neurons in the body.
Almost all interneurons are multipolar (see below).
Motor (efferent) neurons carry impulses or motor commands away from
the central nervous system to effectors/target organs (e.g. muscles and glands). Most
motor neurons have dendrites and cell bodies in the CNS and axons that exit the CNS to
form peripheral nerves that travel to effectors (targets).
The structural classification of neurons is based on the number of processes that extend
from the soma. There are 4 basic neuronal structures like those shown in the figure
below though there are many subtle variations on each theme.
 Neurons can be classified according to their
structure. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Neurons can be classified according to their
structure. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Bipolar neurons have a single dendrite extending from one side of
the cell body and a single axon extending from the other side. Bipolar neurons are small
cells, typically extending for less than 30 microns from dendrite to axon terminal.
There are not many true bipolar cells in the body. A few examples are found in the
special sense organs for vision and olfaction (smell).
Unipolar or pseudounipolar neurons have a single process that
emanates from the cell body. The single process has dendrites on one end and the rest of
the process is an axon. Most sensory neurons of the peripheral nervous system are
unipolar neurons. The dendrites are located in the periphery, where stimuli are
detected. The sensory information travels on the dendrite toward the soma
(usually located ganglia just outside the CNS).. The axon stretches into the CNS at the spinal cord.
Multipolar neurons have two or more dendrites on one side and a
single axon on the other side of the soma. Multipolar neurons are the most common
neurons in the CNS. One example are motor neurons which have dendrites and somas located
in the spinal cord and axons that leave the CNS to innervate skeletal muscles.
Anaxonic neurons are small, stellate (star-shaped) cells with
processes that all look alike with no apparent axon. Anaxonic neurons can be found in
the central nervous system, the retina, and in the adrenal medulla. Their functions are
not well understood.
Most neurons are surrounded by glial cells (neuroglia), the other cell type found in the
nervous tissue. Glial cells are the supportive cells of the nervous system and are 10
times more numerous than neurons. The most well defined role for neuroglia is to provide
structure to the delicate nervous tissue. They fill the space between neurons, serving
as mortar or “glue” and thus hold nervous tissue together. Unlike neurons, glial cells
retain the ability to divide throughout one’s lifetime. When neurons are injured,
neuroglia are stimulated to divide and form glial scars. Glial cells have different
shapes and sizes and their processes are indistinguishable in contrast to the distinct
axon and dendrites found in neurons.
There are 6 types of glial cells, 4 types are found in the CNS and 2 types in the PNS.
The CNS neuroglia are: astrocytes; oligodendrocytes; microglia, and ependymal cells. The 2 types of glia found only in the peripheral nervous
system (PNS) are satellite cells and Schwann
cells.
 There are 6 types of glial cells: 4 found in CNS
and 2 in the PNS. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
There are 6 types of glial cells: 4 found in CNS
and 2 in the PNS. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
CNS Glial Cells
Astrocytes are star-shaped neuroglia and are the most numerous cells in the central
nervous system. They make up half of all cells in the brain. Astrocytes provide a
structurally supportive framework for neurons with their processes wrapping most
non-synaptic regions of neurons in gray matter and covering the entire outer surface of
the brain to form the glial – pia (connective tissue meninx) interface. Astrocytes help
form the protective blood-brain barrier by encircling CNS capillary endothelial cells
and stimulating the cells to form tight-junctions. They help to maintain the
concentration of chemicals in the extracellular space and remove excess signaling
molecules. Astrocytes also react to neural tissue damage by forming scar tissue in the
damaged space.
Oligodendrocytes are glial cells of the CNS that wrap and insulate
axons and give the CNS white matter its characteristic glossy, white appearance.
Oligodendrocytes have a large soma with up to 15 processes. The processes reach out to
axons of nearby neurons and wrap around them (like wrapping tape around a pencil)
forming a high resistance sheath called myelin.
Myelin insulates a small region of the axon (prevents ions from leaking out into
the extracellular fluid), which facilitates signal propagation down the axon towards the
synaptic terminal. A single oligodendrocyte’s processes will wrap axons of numerous
different neurons. Processes from many different oligodendrocytes contribute to the
myelin sheath of a single neuron’s axon.
Microglia are small highly mobile, phagocytic neuroglia that
protect nervous tissue from pathogen infection, remove debris and waste, and may play a
role in remodeling of the synapse that occurs during development and with learning.
About 10-15% of CNS glial cells are microglia. Microglia are derived from monocytes and
thus are more closely related to white blood cells than to the other glial cells. Since
cells of the immune system cannot penetrate the blood brain barrier, microglia serve as
brain macrophages, destroying foreign invaders, promoting inflammation and destroying
cancer cells and cells infected with virus. Clusters of microglia in nervous tissue
provide pathologists with evidence of recent injury.
Ependymal cells are cuboidal-shaped glial cells that are joined
together to form a continuous sheet lining the fluid-filled ventricles and central canal
of the brain and spinal cord. Ependymal cells produce and secrete cerebrospinal fluid
(CSF), the fluid that bathes the tissues of the CNS. The basal side of the cell has
rootlets that anchor the cells to the underlying tissue. The apical surface is marked by
cilia, which helps circulate the CSF.
PNS Glial Cells
The remaining two glial cells, Schwann cells and satellite cells, are found solely in the
peripheral nervous system.
Schwann cells are analogous in function to oligodendrocytes (found
in the CNS). They insulate the axons of peripheral nerves in one of two ways. A Schwann
cell can wind its way round and round the axon (up to 100 times), while squeezing its
cytoplasm out of the way (much like a toothpaste tube could be wrapped around a pencil),
forming a myelin sheath. Like myelinating a single fiber in the CNS, which requires many
oligodendrocytes, a complete myelin sheath in the PNS requires many Schwann cells.
Schwann cells can also envelop PNS axons without forming a myelin sheath. Instead of
wrapping a single axon many times, the Schwann cell forms an envelope around a bundle of
unmyelinated axons.
Additionally, Schwann cells can also assist in the regeneration of a damaged peripheral
nerve. If a peripheral nerve is damaged, it may regenerate if its soma is undamaged and
the neurilemma (the plasma membrane of the Schwann cell)
enveloping it is intact.
Satellite cells are found surrounding neural somas in peripheral
ganglia (collections of cell bodies located outside the CNS).
Satellite cells resemble CNS astrocytes and are thought to have similar functions,
providing structural support and regulating the chemical environment.
Neuronal axons in the CNS and PNS can be devoid of a glial cell wrap
(unmyelinated) or they can be discontinuously wrapped by glial cells along their entire
length (myelinated). In a myelinated axon, the bare regions where the sheath is
interrupted are called Nodes of Ranvier. The myelinated segments
between consecutive nodes of Ranvier are called internodes. The
myelin sheath changes the appearance of axons as well as their electrical properties.
Myelinated axons appear white when viewed by the naked eye in contrast to areas where
neuronal cell bodies are concentrated which appear gray.
Neurons produce electrical signals as a way of conveying information from one place in
the body to another place very quickly, at speeds up to 100 meters/second (200 miles per
hour). These rapidly traveling electrical signals allow you to perceive sensory stimuli,
like the sound of a passing fire truck blasting its siren. Electrical signals,
travelling in different neural pathways, coordinate motor responses that allow you to
move your car out of the way of the fire truck, withdraw your hand from a dangerously
hot pan, and rhythmically contract your diaphragm to breathe. Electrical signals arise
as a result of movement of ions back and forth across the cell membrane of neurons. As
ions move down their electrochemical gradients, they carry their charge with them,
creating very miniscule but physiologically important electrical currents. These ionic
currents flowing across membranes are the basis for the propagating electrical signals
that underlie all nervous system functions. In this section, we’ll explore how neurons
generate these electrical signals.
All living, eukaryotic cells have a transmembrane potential (a
difference in charges between the intracellular and extracellular fluid). While the cell is at rest (i.e., unstimulated), the transmembrane potential
is stable and is called the resting membrane potential (RMP).
Right at the cell membrane, there is a little excess negative charge
on the inside of
the cell membrane and a little excess of positive charge on the
outside. Because
separation of charges creates a voltage, a very small probe on a
voltmeter can be used
to measure the voltage across the cell membrane. By convention the
voltage outside the
cell is set to zero. In a typical cell, the voltage recorded across
the membrane is between -60 and -90 millivolts(-.06 to -.09 volts) with
the negative sign indicating that the
inside of the cell is negative with respect to the outside. Some
cells have the ability
to transiently alter their transmembrane potential (excitable cells),
while others do
not (non-excitable cells).
Non-excitable cells (ex: intestinal epithelial cells) have a stable
and unchanging RMP. Excitable cells, like neurons and muscle, have
a membrane potential that can fluctuate under certain conditions, with each fluctuation
representing a signal produced by the cell. These fluctuations may be small and local to
a region of a cell membrane (often called local or graded
potentials) or larger in magnitude and travel along the length of the cell.
These latter potentials, called action potentials, always lead to
some response by the cells. In a neuron, action potentials lead to neurotransmitter
release.
 The resting
membrane potential (measured as a voltage) is an energy gradient across the cell
membrane due to a slight separation of charge right along the cell membrane. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The resting
membrane potential (measured as a voltage) is an energy gradient across the cell
membrane due to a slight separation of charge right along the cell membrane. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
To understand the basis of the resting membrane potential, we must first investigate the
composition of body fluids - intracellular fluid (ICF) and extracellular fluid (ECF).
Recall that ICF and ECF are composed of salts like NaCl and KCl that dissociate into
their ionic components when placed in aqueous solutions. These ions are the mobile
charges in body fluids that move between compartments to generate electrical currents
along cell membranes.
 NaCl dissociates in water to form ions, mobile charge
carriers used to generate electrical currents in living organisms. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
NaCl dissociates in water to form ions, mobile charge
carriers used to generate electrical currents in living organisms. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The ECF and ICF have high concentrations of different salts, which leads to differing
concentrations of individual ions in the ECF versus the ICF. Recall from your earlier
studies that sodium (Na+), chloride (Cl-), and calcium
(Ca2+) are more highly concentrated in the ECF, while potassium
(K+) and large anions (An-) are more highly concentrated in
the ICF.
| Ionic Composition of the ECF versus the ICF |
|---|
| This table
does not include all the ions, but only those that are major contributors to the
membrane potential and changes in the membrane potential. |
| Ion |
Extracellular Fluid (mM) |
Intracellular Fluid (mM) |
| K+
|
5 |
150 |
| Na+
|
145 |
15 |
| Cl-
|
108 |
10 |
| Ca2+
|
1 |
0.0001 |
|
The resting membrane potential arises due to the combined effects of three factors, which
determine what ions move across the cell membrane in an unstimulated cell (at rest).
Ions are not evenly distributed between the inside and the outside of a cell. As we just
learned, sodium is nearly 10 times more concentrated outside the cell than inside.
Conversely, potassium is nearly 30 times more concentrated inside the cell than outside.
The uneven distribution of ions leads to concentration gradients across the cell
membrane. Given the opportunity, ions will move down their concentration gradient (i.e.,
from an area where they are highly concentrated to an area where they are less
concentrated). So, given the chance, sodium ions would move into the cell and potassium
ions would move out of the cell based on their respective concentration gradients.
The ionic composition of body fluids is tightly regulated. Small increases or decreases
in ion concentrations can disrupt normal functions of the brain, heart, skeletal muscle
or other organs. In many instances, you or one of your loved ones may need to receive
treatment for an electrolyte imbalance. These situations might be as simple as that
restoring blood lost in an accident, or receiving fluids to rehydrate you following a
soccer tournament. The article below shows how critically important it is that people
receive solutions with appropriate ion concentrations in their treatments.
Example
Let’s review a tragic case where simple calculations to double check a dose of CaCl2
given to a baby to restore body fluids, might have saved her life.
Baby dies at Seattle Children's hospital after overdose
An infant in the intensive-care unit of Seattle Children's hospital died after she was
administered 10 times the dose of a medication, calcium chloride, by a hospital nurse,
according to a notice sent by hospital CEO Tom Hansen to the staff.
By Carol M. Ostrom, Seattle Times health reporter
Eight-month-old Kaia Zautner was in the intensive-care unit of Seattle Children's
hospital, battling back from serious heart problems and surgeries, when a hospital nurse
gave her 10 times the proper dose of a medication, calcium chloride.
Five days later, on Sept. 19, after suffering a brain hemorrhage, the baby died.
Tom Hansen, hospital CEO, in a notice to staff on Sept. 22, said the hospital has offered
"heartfelt apologies" to the family, without naming them. "This was a catastrophic
outcome for the patient and the family, and caused serious distress for staff members as
well," Hansen said.
In a family blog, Kaia's parents, Jared and Alana Zautner, of Puyallup, had described
their baby's fight to overcome the heart problem she'd had since her birth on Jan. 12
and then, just days after her "8th month birthday," the "horrible turn of events" that
gave them "one of the scariest days of our lives."
The overdose was an accidental miscalculation, Alana Zautner wrote on the blog, thanking
friends for their continued prayers.
"I have seen such strength in my daughter these last few hours and I have faith that she
will pull through this," she wrote. "I just pray for a miracle and that she will be
completely touched and healed."
A memorial for Kaia was held Saturday at Lighthouse Christian Center (Alana wrote that
all doctors and nurses were welcome), and a "Hawaii Lei Ceremony and Scattering of the
Ashes" is planned for Oct. 2 on Maui, Hawaii.
The hospital, as required, reported the overdose to the state Department of Health, which
collects statistics on "adverse events" in hospitals.
The hospital reviewed the clinical record after the overdose and began a detailed
analysis of why the usual safety checks had not prevented it, Hansen said in the letter
to staff.
"Perhaps the best tribute we can pay to this family is by doing everything we can to
prevent future medical errors in our system," Hansen said in the letter. "An important
way we can make medicine safer is if we admit that mistakes occur and openly investigate
them. We must learn from these events and work together to evaluate our processes and to
error-proof our care processes."
Hansen said it is personally important to him that all staff and faculty feel safe to
report mistakes.
While the investigation is under way, he said, the hospital will allow only pharmacists
and anesthesiologists to fill needles with calcium chloride in nonemergency situations,
but the drug can still be accessed by medical or nursing staff if needed in an
emergency.
Hansen did not say whether the nurse who administered the overdose was disciplined by the
hospital.
The state's Nursing Care Quality Assurance Commission has also opened an investigation,
according to Department of Health spokesman Tim Church.
"We don't even have a name yet," he said. "The nursing commission is opening (the
investigation) because it's aware of the situation — not under any particular name."
The hospital will have 45 days to complete a "root cause" analysis of the event, Church
said, but that report will not be publicly available.
In 2009, a 15-year-old Kent boy died after using a painkilling patch prescribed by his
dentist at Children's. The boy, Michael Blankenship, had four teeth extracted at the
hospital and was sent home with the pain patch containing Fentanyl, prescribed by his
dentist at the hospital. The teen was found dead the next morning.
The teen was autistic and could not tolerate pills or liquid medicine.
The hospital's medical director said the highly potent narcotics patch should not have
been prescribed.
His family filed a lawsuit against the hospital last September.
Update, 11:32a.m., Sept. 29: The family reached a settlement earlier
this year, but didn't disclose the terms.
Source:
http://seattletimes.nwsource.com/html/localnews/2013016258_infantdeath29m.html?syndication=rss
However, the cell membrane is not freely permeable to ions. Ions cannot freely cross the
plasma membrane because of its structure. The lipid core of the cell membrane is
hydrophobic and does not allow charged molecules to pass through it. Rather the cell
membrane is selectively permeable, meaning it allows certain ions to pass. You know that
the ions do not pass directly through the cell membrane, but rather pass through ion
channels. The membrane is permeable to a specific ion if there are open channels for
that ion. Recall that ion channels open and close based on the presence of electrical or
chemical stimuli. Voltage-gated channels open at specific membrane potentials and are
either inactivated (while the stimulus persists) or close when the membrane potential
changes. Ligand-gated channels open when they bind chemicals and close when the chemical
is no longer bound.
At rest, the cell membrane is most permeable to potassium because there are more open
potassium channels at the resting membrane potential than channels for any other ion. As
a result, potassium “leaks” out of the resting cell. The resting membrane is less
permeable to sodium, and, at rest, a small amount of sodium “leaks” into the cell.
If these were the only things happening in the resting cell, the resting membrane
potential would not be stable, but rather the net movement of potassium ions would cause
the membrane potential to change. Ions move not only based on their individual
concentration gradients, but they also move based on charge attraction and repulsion.
Ions move away from like charges (ex. sodium and potassium ions move away from each
other) and move towards opposite charges (ex. potassium ions would move toward chloride
ions). The net movement of a particular ion is influenced by its electrochemical gradient (the balance of its concentration gradient and any
charge attraction or repulsion).
Example
Electrochemical Gradient
Let’s consider how the electrochemical gradient affects a potassium ion. You’ve already
learned that the RMP ranges from -60 mV to -90 mV in living cells. The negative sign
indicates that the inside of the cell is negatively charged with respect to the outside.
You also know that potassium is 30 times more concentrated on the inside of the cell
than the outside. So, the concentration gradient causes potassium ions to leave the cell
(through open ion channels), but the electrical gradient causes the positively charged
potassium ion to be attracted to re-enter the cell. As a result, potassium ions not only leave
the cell at rest (due to the concentration gradient), but they also re-enter the cell
(due to the electrical gradient). The overall movement of the ion depends on the
strength of each of the two gradients. The concentration gradients are stronger in a
resting cell; therefore, more ions will move in the direction favored by the
concentration gradient than will move based on the electrical gradient.
One final factor also plays a role in determining the RMP. The sodium potassium pump
operates continually in living cells. At maximum capacity, it pumps 3 sodium ions out of
the cell and 2 potassium ions into the cell, and hydrolyzes 1 ATP to provide the energy
for the ion transport.
 The sodium potassium pump transports 3 Na+ to
the ECF and 2 K+ to the ICF. By Mariana Ruiz Villarreal (http://upload.wikimedia.org/wikipedia/commons/thumb/a/a5/scheme_sodium-potassium_pump-en.svg/300px-scheme_sodium-potassium_pump-en.svg.png) Public Domain.
The sodium potassium pump transports 3 Na+ to
the ECF and 2 K+ to the ICF. By Mariana Ruiz Villarreal (http://upload.wikimedia.org/wikipedia/commons/thumb/a/a5/scheme_sodium-potassium_pump-en.svg/300px-scheme_sodium-potassium_pump-en.svg.png) Public Domain.
The sodium potassium pump is electrogenic (there are an uneven
number of charges transported into and out of the cell resulting in a net charge
associated with each exchange cycle). Since 3 sodium ions leave the cell and only 2
potassium ions enter the cell, there is a net negative charge on the inside of the cell
due to the sodium potassium pump.
Neurons can be excited by various stimuli such as light, chemicals, heat or pressure.
These stimuli, which are typically received at the dendrites, cause small, localized
changes in membrane voltage. The stimuli open ion channels in the membrane, which allows
specific ions to flow in or out of the cell. This ion movement produces a change in the
membrane voltage around the area of the open channels. These local shifts in membrane
potential are called local (or graded) potentials. Local potentials have the following
characteristics:
They are graded, which means the change in
membrane voltage that occurs is proportional to the size of the stimulus. A stronger
stimulus can open more ion channels. A stimulus that lasts for a long time can either
open more ion channels or keep channels open for a longer time. In either case, more
ions are able to cross the cell membrane, which produces a larger change in membrane
voltage.
They are decremental, meaning that the
signal grows weaker as it moves farther from the site of stimulation. Ion channels are
opened at the site of stimulation and that is where ions move across the cell membrane.
As a result, there is a high concentration of ions right around the ion channels. Once
the ions cross the membrane, they diffuse away from the channel and there are fewer and
fewer ions as they move away from the open channels. Fewer ions results in a smaller
change in membrane potential.
They are reversible. If the stimulus comes
to an end, the ion channels close and resting membrane potential is re-established
before the signal travels very far.
They can either excite the cell or
inhibit the cell depending on what type of
ion channel is opened. If the stimulus opens a sodium channel, sodium enters the cell
and deporlarizes (make the membrane potential less negative) the
membrane around the open channels. If the stimulus opens a chloride channel, chloride
ions enter the cell and make the local membrane potential more negative than the RMP
(hyperpolarizes the cell). Depolarization excites the cell and
makes it more likely to send a signal to other cells. Hyperpolarization inhibits the
cell and makes it less likely to send a signal to other cells.
A stimulus can also affect potassium channels. If the stimulus causes potassium channels
to open, the effect will be hyperpolarization of that area of cell membrane. Potassium
leaves the cell through the open channels, which removes positive charges from the ICF
making the inside of the cell more negative. If the stimulus closes potassium channels,
the membrane will depolarize around the closed channels because fewer potassium ions are
leaving the cell.
Neurons generally receive multiple stimuli at the same time – some may be excitatory and
others inhibitory. The overall response of the neuron will depend on the net effect of
all the stimuli. In some cases, the neuron will produce a signal that will travel to
other cells. In other cases, no signal will be sent from the neuron.
If there is adequate excitatory stimulation of a neuron, a signal called an action potential is generated. An action potential is a transient
and marked shift in membrane potential that occurs when voltage-gated ion channels in
the membrane open. A series of action potentials can rapidly carry information from the
neural soma along the axon to the axon terminal. A sufficient number of voltage-gated
channels must be present in the cell membrane to initiate an action potential. The
dendrites and most of the soma lack enough voltage-gated ion channels for this. However,
at the trigger zone, where the soma interfaces with the axon,
there is a high concentration of voltage-gated channels. To create an action potential
in a neuron, an excitatory local potential must reach the trigger zone and depolarize (a shift in membrane potential making it less negative
or even positive) it to the threshold voltage needed to open the ion channels.
Two types of voltage-gated channel are responsible for the propagating action potentials
in most neurons – a fast Na+ channel (a voltage-gated Na+ channel that opens quickly
when stimulated) and a slow K+
channel (a voltage-gated K+ channel that
opens slowly when stimulated) . Let’s take a closer look at the specific events of an
action potential.
Excitatory local potentials reach the trigger zone and depolarize it. If the local
potentials depolarize the membrane to threshold (the membrane
voltage at which the voltage-gated channels are stimulated to open), these voltage-gated
channels begin to open. The fast Na+ channel opens quickly, increasing the
permeability of the membrane to Na+ that flows into the cell down
its electrochemical gradient leading to further depolarization. This causes more fast
Na+ channels to open, further depolarizing the membrane. As the membrane
potential reaches 0 mV, the fast Na+ channels become “inactivated.” A second
gate that works like a timer closes the channel. By the time all the fast Na+
channels are inactivated the membrane voltage has reached its peak.
As the fast Na+ channels are being inactivated, the slow K+
channels are finally opening. This increases the permeability of the membrane to
K+. Potassium ions leave the cell moving down their electrochemical
gradient, and the efflux of positive charge causes the membrane voltage to return toward
the resting membrane potential (repolarization).
Slow K+ channels stay open longer than fast Na+ channels, so more
K+ leaves the cell than Na+ entered. The removal of excess
potassium ions causes the membrane potential to become more negative than the resting
membrane potential. When this happens, we say the membrane is hyperpolarized.
The changes in membrane permeability and their relationship to the membrane potential can
be seen in the following figure.
The duration of time that the membrane is hyperpolarized following an action potential is
termed its refractory period. The refractory period is an interval
of time during which that part of the membrane cannot be excited (to produce another
action potential) or requires a larger than normal stimulus to be excited. The
refractory period is divided into two parts based on whether or not the membrane can be
stimulated to produce an action potential. During the absolute
refractory period, the membrane cannot be stimulated to produce another action
potential regardless of the strength of the stimulus. During the relative refractory period, the membrane can be stimulated to produce an
action potential, but a stronger than normal stimulus is required.
The absolute refractory period lasts from the beginning of the action potential (when the
membrane reaches the threshold voltage) until the fast Na+ channels reset to
their resting state. As long as the Na+ channels are open or inactivated, a
new action potential cannot be generated.
The relative refractory period continues from the end of the absolute refractory period
until the membrane is no longer hyperpolarized (returns to the resting membrane
potential). During hyperpolarization, slow K+ channels are still open, but
are in the process of closing. In order to stimulate an action potential during this
time, a very strong stimulus is needed to overcome the effect of potassium flowing out
of the cell and depolarize the cell.
Previously, we considered the characteristics of local potentials. They are graded,
decremental, reversible, and can either excite or inhibit the membrane. In contrast,
action potentials are all-or-none, nondecremental, irreversible and always
excitatory.
Action potentials within a particular cell are all identical regardless of stimulus
strength. If the membrane at the trigger zone reaches the threshold voltage or a voltage
above the threshold, a maximal action potential will be generated. If the threshold
voltage is not attained, no action potential is generated (no signal is propagated). In
this way, action potentials are all-or-none – a cell either fires
a full action potential or no action potential at all.
The action potential at the axon terminal looks exactly like the action potential that
was initially generated at the trigger zone. Since the signal does not change as it
travels the length of the axon it is nondecremental. It should be
noted that the action potential at the axon terminal is not the same one that originated
at the trigger zone. Rather, a series of identical action potentials are generated as
the signal travels toward the axon terminal.
If the membrane reaches threshold, an action potential will be initiated and the signal
will be propagated down the entire axon. Once the events are set in motion there is no
stopping them. The process is irreversible.
In contrast to local potentials, which can either excite or inhibit the membrane, action
potentials are all excitatory (cause an initial depolarization of the membrane).
Several neurons are generally needed to transmit a signal from one place in the body to
another. So how does the signal pass from one neuron to the next along a neural pathway?
The signal must be transmitted across the interface between successive neurons and we
will learn how that is accomplished in this next section.
 Synapse cells. By Miserlou at en.wikipedia (http://upload.wikimedia.org/wikipedia/commons/3/3e/Neurons_big1.jpg)
Synapse cells. By Miserlou at en.wikipedia (http://upload.wikimedia.org/wikipedia/commons/3/3e/Neurons_big1.jpg)
The term synapse means “coming together.” Where two structures or entities
come together, they form a synapse. Although one can use the word synapse to mean any
cellular junction, in physiology we traditionally limit its usage to: the junction of
two neurons, the junction between a neuron and a target cell (ex. the neuromuscular
junction), or the interface between adjacent cardiac muscle cells or adjacent smooth
muscle cells. In the nervous system, a synapse is the structure that allows a neuron to
pass an electrical or chemical signal to another cell.
The cell that delivers the signal to the synapse is the presynaptic cell. The cell that will receive the signal once it
crosses the synapse is the postsynaptic cell. Since most
neural pathways contain several neurons, a postsynaptic neuron at one synapse
may become the presynaptic neuron for another cell downstream.
A presynaptic neuron can form one of three types of synapses with a postsynaptic
neuron. The most common type of synapse is an axodendritic synapse, where the
axon of the presynaptic neuron synapses with a dendrite of the postsynaptic
neuron. If the presynpatic neuron synapses with the soma of the postsynaptic
neuron it is called an axosomatic synapse, and if it synapses with the axon of
the postsynaptic cell it is an axoaxonic synapse. Although our illustration
shows a single synapse, neurons typically have many (even 10,000 or more)
synapses.
There are two types of synapses found in your body: electrical and chemical. Electrical
synapses allow the direct passage of ions and signaling molecules from cell to cell. In
contrast, chemical synapses do not pass the signal directly from the presynaptic cell to
the postsynaptic cell. In a chemical synapse, an action potential in the presynaptic
neuron leads to the release of a chemical messenger called a neurotransmitter. The neurotransmitter then diffuses across the synapse and
binds to receptors on the postsynaptic cell. Binding of the neurotransmitter leads to
the production of an electrical signal in the postsynaptic cell.
Why does the body have two types of synapses? Each type of synapse has functional
advantages and disadvantages. An electrical synapse passes the signal very quickly,
which allows groups of cells to act in unison. A chemical synapse takes much longer to
transmit the signal from one cell to the next; however, chemical synapses allow neurons
to integrate information from multiple presynaptic neurons, determining whether or
not the postsynaptic cell will continue to propagate the signal. Neurons respond differently
based on information transmitted by multiple chemical synapses. Let’s take a
closer look at the structure and function of each type of synapse.
Electrical synapses transmit action potentials via the direct flow
of electrical current at gap junctions. Gap junctions are formed
when two adjacent cells have transmembrane pores that align. The membranes of the two
cells are linked together and the aligned pores form a passage between the cells.
Consequently, several types of molecules and ions are allowed to pass between the cells.
Due to the direct flow of ions and molecules from one cell to another, electrical
synapses allow bidirectional flow of information between cells. Gap junctions are
crucial to the functioning of the cardiac myocytes and smooth muscles.
 Structure of an electrical synapse (gap junction). By (https://upload.wikimedia.org/wikipedia/commons/thumb/b/b/b7/Gap_cel_junction-en.svg/500px-Gap_cell_junction-en.svg.png).
Structure of an electrical synapse (gap junction). By (https://upload.wikimedia.org/wikipedia/commons/thumb/b/b/b7/Gap_cel_junction-en.svg/500px-Gap_cell_junction-en.svg.png).
Chemical synapses comprise most of the synapses in your body. In a
chemical synapse, a synaptic gap or cleft
separates the pre- and the postsynaptic
cells. An action potential propagated to the axon terminal results in
the secretion
of chemical messengers, called neurotransmitters, from the axon
terminals. The neurotransmitter molecules diffuse across the synaptic
cleft and bind to receptor proteins on the cell membrane
of the postsynaptic cell. Binding of the neurotransmitter to the
receptors on the postsynaptic cell leads to a transient change in the
postsynaptic
cell’s membrane potential.
 Structure of a chemical synapse. (http://creativecommons.org/licenses/by/3.0/us/).
Structure of a chemical synapse. (http://creativecommons.org/licenses/by/3.0/us/).
The process of synaptic transmission at a chemical synapse between
two neurons follows these steps:
An action potential, propagating along the axon of a presynaptic neuron, arrives at the
axon terminal.
The depolarization of the axolemma (the plasma membrane of the
axon) at the axon terminal opens Ca2+ channels and Ca2+ diffuses
into the axon terminal.
Ca2+ bind with calmodulin, the ubiquitous intracellular
calcium receptor, causing the synaptic vesicles to migrate to and
fuse with the presynaptic membrane.
The neurotransmitter is released into the synaptic cleft by the process of exocytosis.
The neurotransmitter diffuses across the synaptic cleft and binds
with receptors on the postsynaptic membrane.
Binding of the neurotransmitters to the postsynaptic receptors causes a response in the
postsynaptic cell.
The response can be of two kinds:
- A neurotransmitter may bind to a receptor that is associated with a specific ion-channel
which, when opened, allows for diffusion of an ion through the channel. If Na+
channels are opened, Na+ rapidly diffuses into the postsynaptic cell
and depolarizes the membrane towards the threshold for an action potential. If K+
channels are opened, K+ diffuses out of the cell, depressing the
membrane polarity below its resting potential (hyperpolarization). If Cl-
channels are opened, Cl- moves into the cell leading to
hyperpolarization.
- The neurotransmitter may bind to a transmembrane receptor protein, causing it to activate
a G-protein on the inside surface of the postsynaptic membrane. A cascade of events
leads to the appearance of a second messenger (calcium ion, cyclic AMP (cAMP), or
IP3) in the cell. Second messengers can have diverse effect on the cell
ranging from opening an ion channel to changing cell metabolism to initiating
transcription of new proteins.
The response of the postsynaptic cell to a neurotransmitter depends on the specific
receptors that are present on its cell membrane. Most neurotransmitters can bind to more
than one receptor found in the body and the cell’s response is dependent on which
receptor is bound. Different receptors produce different cellular responses because they
activate processes in the cell.
Example
Cholinergic Receptors
Let’s look at an example. Receptors that can bind the neurotransmitter acetylcholine
(ACh) are termed cholinergic receptors – this is the type of receptor. There is more
than one cholinergic receptor – the different cholinergic receptors are termed subtypes.
One subtype, the nicotinic cholinergic receptor, opens a sodium channel when it binds
ACh. Stimulation of a nicotinic cholinergic receptor leads to depolarization of the
cell. Another subtype, the muscarinic cholinergic receptor, opens a potassium channel
when it binds ACh. Stimulation of a muscarinic cholinergic receptor leads to cell
hyperpolarization. Acetylcholine can either excite or inhibit the postsynaptic cell
depending on whether that cell has the nicotinic or muscarinic receptor subtype.
 Cholinergic receptors that open ion channels cause changes
in membrane potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Cholinergic receptors that open ion channels cause changes
in membrane potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
In the example we just considered, both receptor subtypes were linked to distinct ion
channels. It is also possible for one receptor subtype to be linked to an ion channel
while another subtype leads to the production of a second messenger. In this case, the
timing of the postsynaptic cell’s response is different. Opening an ion channel takes
very little time compared to the complex signaling that occurs with a second messenger.
The response is fast with a receptor linked to an ion channel and is slow with a
receptor that leads to a second messenger cascade. Although slower, second messenger
cascades can produce more diverse cellular effects and have the advantage of amplification.
Binding of a single molecule of neurotransmitter can produce many molecules of the
second messenger. In contrast, if the receptor opens an ion channel, a single molecule
of neurotransmitter (or sometimes two molecules) is needed to open a single ion channel
in the postsynaptic cell.
 A receptor that produces a second messenger in the
postsynaptic cell. Second messengers can lead to a wide range of effects in the
postsynaptic cell. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
A receptor that produces a second messenger in the
postsynaptic cell. Second messengers can lead to a wide range of effects in the
postsynaptic cell. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Postsynaptic potentials develop in the postsynaptic cell’s membrane when neurotransmitter
binding to receptors leads to the opening of ion channels. An excitatory postsynaptic potential (EPSP) occurs if the membrane is depolarized
by the ion movement. If, on the other hand, the membrane becomes
hyperpolarized when the ions move, an inhibitory postsynaptic potential
(IPSP) is generated. EPSPs and IPSPs are local potentials.
EPSP: Opening of sodium- or calcium channels leads to
depolarization of the membrane. If there is sufficient depolarization, the threshold potential is reached and an action
potential will be produced in the postsynaptic membrane.
Since an EPSP depolarizes the membrane, it facilitates action
potentials.
 An excitatory postsynaptic potential depolarizes the
membrane bringing it closer to the threshold potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
An excitatory postsynaptic potential depolarizes the
membrane bringing it closer to the threshold potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
IPSP: Opening of potassium- or chloride channels leads to
hyperpolarization of the membrane. (Since the current is outward for potassium ions, and
inward for chloride ions, opening of either of these two channels will cause the
postsynaptic membrane to hyperpolarize.) A hyperpolarized membrane has moved farther
from the threshold potential and has less probability of producing an action potential.
Since an IPSP hyperpolarizes the membrane, it inhibits action
potentials.
 An inhibitory postsynaptic potential hyperpolarizes the
membrane taking it farther from the threshold potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
An inhibitory postsynaptic potential hyperpolarizes the
membrane taking it farther from the threshold potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Remember that a neuron synapses with many other neurons. So a postsynaptic neuron can
receive signals from many presynaptic neurons simultaneously. Whether or not the
postsynaptic cell has an action potential depends on the summation (the additive effect)
of all the incoming signals. Each active synapse can result in a local potential (either
an EPSP or an IPSP). The net effect of all the local potentials on the trigger zone
determines whether or not there is an action potential in the postsynaptic cell.
There are two different ways that local potentials can sum to excite the postsynaptic
cell to have an action potential. Temporal summation occurs when
successive EPSPs at a single synapse occur in rapid succession. The successive
potentials occur before the previous ones die out producing an increasing membrane
depolarization.
 Temporal summation occurs when one synapse stimulates the
postsynaptic cell very quickly and the EPSPs produced in the postsynaptic cell piggyback
on each other causing an increasing level of depolarization. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Temporal summation occurs when one synapse stimulates the
postsynaptic cell very quickly and the EPSPs produced in the postsynaptic cell piggyback
on each other causing an increasing level of depolarization. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
 The effect of temporal summation on membrane voltage at
the trigger zone. The threshold voltage is attained and the postsynaptic cell fires an
action potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The effect of temporal summation on membrane voltage at
the trigger zone. The threshold voltage is attained and the postsynaptic cell fires an
action potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Summation can also occur when multiple presynaptic neurons stimulate the postsynaptic
neuron at the same time (spatial summation). Each individual
synapse lets in a limited number of ions and alters the membrane potential a little. The
collective effect of all the synapses allows in enough ions to reach the threshold
potential and an action potential is triggered.
 Spatial summation occurs when the collective effect of
multiple synapses depolarizes the postsynaptic neuron to threshold resulting in an
action potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Spatial summation occurs when the collective effect of
multiple synapses depolarizes the postsynaptic neuron to threshold resulting in an
action potential. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The interplay between IPSPs and EPSPs is
important. Whether an action potential is going to be produced
depends not just on the
summation of the EPSPs, but on the summation of EPSPs and IPSPs.
The algebraic sum of all EPSPs and IPSPs has to be of sufficient
amplitude to raise
the membrane potential to the threshold for an action potential.
What this means is that if the IPSPs prevail, then the post-synaptic
cell will be
“silent.” One can, therefore, visualize the process of summation
as a “tug-of-war” between excitatory and inhibitory currents induced
by the binding of neurotransmitters on excitatory or inhibitory
postsynaptic receptors, respectively.
Neurotransmitters are organic molecules that allow neurons to communicate
with each other and with target cells. Neurotransmitters fall into four classes based on
their chemical makeup.
Acetylcholine (ACh) is a small molecule formed from acetate and
choline. It is in a class by itself. Acetylcholine is the sole neurotransmitter used at
the neuromuscular junction and is also the neurotransmitters used by the parasympathetic
nervous system.
 The structure of acetylcholine.
The structure of acetylcholine.
Some amino acids act as neurotransmitters. Glycine and
γ-aminobutyric acid (GABA) are the most common inhibitory neurotransmitters in the
spinal cord and brain, respectively. Glutamate (glutamic acid) and aspartate are
excitatory neurotransmitters found in the brain and spinal cord, respectively.
Biogenic amines (monoamines) are formed from amino acids from which
the carboxyl terminus is removed. Three of the biogenic amines, called the catecholamines, are grouped together as they are all derived from
the same amino acid, L-tyrosine. The catecholamines include: norepinephrine (noradrenalin), epinephrine (adrenalin)
and dopamine (dopamine can also be made from phenylalanine).
Norepinephrine (NE) is the neurotransmitter of the sympathetic nervous system (your
fight or flight response). Epinephrine (E) has similar effects to NE, but is less
abundant. Dopamine is best known for its role in motor inhibition. Loss of dopamine
producing neurons in Parkinson disease leads to dyskinesias (movement disorders).
Catecholamines bind to adrenergic receptors. Other biogenic amines include serotonin and
histamine.
 The structures of the biogenic amine
neurotransmitters. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The structures of the biogenic amine
neurotransmitters. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Neuropeptides are small proteins that function as
neurotransmitters. They are the largest neurotransmitters and can range from just a
couple of amino acids to as many as 40. An example of neuropeptide neurotransmitters is
β-endorphin, the chemical associated with the elevated mood experienced with
exercise.
 Examples of neuropeptide neurotransmitters. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Examples of neuropeptide neurotransmitters. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Example
The Autonomic Nervous System at Work
Josie is sitting, having an outdoor lunch with friends when a large spider lands on
her plate. She immediately freezes, the food in her mouth begins to feel like
a wad of dry hay, and she nearly gags as she tries to swallow it. She feels her
heart race and pound in her chest. After swallowing her food, it seems stuck in her
throat and chest.
In this scenario, Josie had been sitting, relaxed and enjoying a meal. In this relaxed
state, the body would have a heart rate that is at rest, active peristalisis
(activity in muscles of the digestive system), ample activity in
salivary glands and in digestive gland secretions, and bronchi that are not dilated.
In this relaxed state, food can be easily processed due to ample amounts of saliva
and digestive enzymes in the saliva released by the salivary glands into the mouth.. Salivation also facilitates
swallowing by providing lubrication to the back of the throat and the esophagus. In
this relaxed state, digestive fluids and enzymes in the intestines are actively
produced and secreted so that food can be further processed and broken down
(catabolized) for absorption of nutrients and glucose. While relaxed, Josie's heart
beats imperceptibly and her breathing is deep.
With the sudden appearance of the spider, the rate of Josie's heart beat becomes more
rapid, and it contracts more powerfully. In this vigilant state, Josie senses her
rapid heart rate as well as the increased force of the contraction of her heart. She
also senses a shift to rapid, shallow breathing that she tries to control. Her food
seems lodged near the back of her throat as she struggles to swallow her food
safely.
Within seconds of seeing the spider, Josie's body systems shifted from reflecting
calm to a state of panic and hyper-vigilance. These responses to the
external threat prepare Josie to either fight or flee the situation. In the
hyper-vigilant state, Josie's pupils widen, she sweats and more blood is pumped
through her blood vessels which permits more blood, chemicals, and hormones to flow
to her skeletal muscles and respiratory system. As you can see, the effects on organ
systems when in either of the states, relaxed or hyper-vigilant, are nearly
opposite. These two states are controlled by two subsets of neural pathways that are
part of the peripheral nervous system.
The peripheral nervous system itself has two main functional parts. These
are the somatic nervous system, which controls voluntary movements of
skeletal muscles, and the autonomic nervous system, which is the
involuntary motor division of the nervous system.
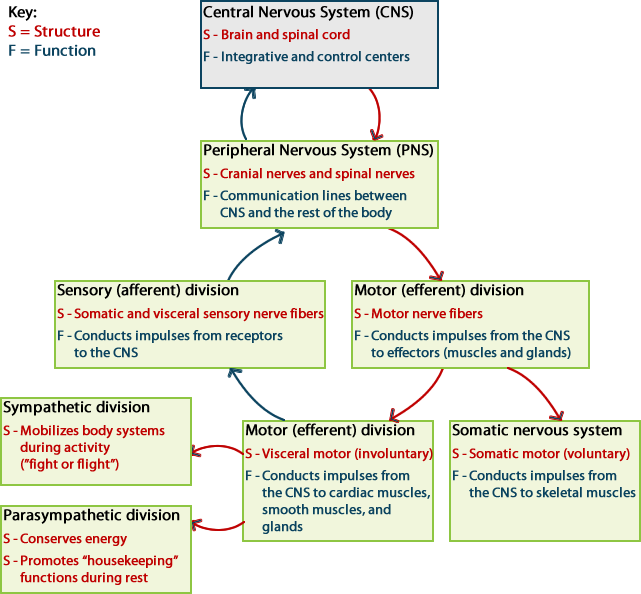 The central nervous system and its divisions
The central nervous system and its divisions
The
autonomic nervous system is further divided into the sympathetic nervous system and the
parasympathetic nervous system. It is the complementarity of these two
latter branches of the autonomic nervous system that drove the physiological changes in
the “Spider Sat Down Beside Her” scenario above.
As evident from the impact of the sight of the spider on Josie's ability to eat her meal,
activity in the parasympathetic system is associated with a relaxing meal;
on the other hand, activity in the sympathetic system is associated with
alertness and vigilance. In keeping with the complementary functionality of the two
systems, they have been given nick names. The parasympathetic branch works for
“rest and repose” (also commonly known as “rest and digest”); the
sympathetic branch is known for the “fight or flight” response (variously also known as
“fight, flight or freeze;” “hyperarousal;” “acute stress”).
It will also be evident in later sections that both parasympathetic and
sympathetic branches influence most organs, typically in opposition to
each other. However, as will also be clear from the discussion going forward,
physiologically this opposition is more complementary than antagonistic. One can give an
example of a car, where the accelerator and brake are both necessary for its operation.
The balancing that goes on between these two divisions of the ANS requires that most
organs receive inputs from both. This is referred to as dual innervation.
We will see examples of this later.
The peripheral nervous system is divided into two functional subdivisions:
- Somatic Nervous System (SNS)
- Autonomic Nervous System (ANS)
The autonomic nervous system is further divided into the sympathetic and
parasympathetic branches.
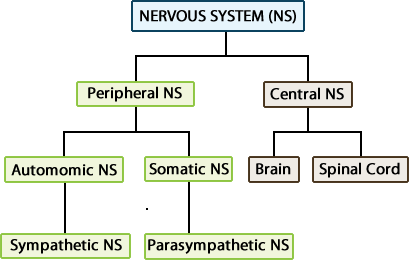 Division of the Nervous System into Peripheral NS and Central NS.
Division of the Nervous System into Peripheral NS and Central NS.
The somatic nervous system (SNS) deals with sensory input and voluntary motor
(efferent) activities, while the autonomic nervous system (ANS) deals only with
efferent (motor) signals from the CNS to control activities in the body that are
distinct from those under conscious voluntary control. The targets of efferents are
called effectors, and these are organs, muscles or glands. The autonomic
nervous system is also called the visceral nervous system because it controls smooth
muscle, cardiac muscle, and glands, which make up the viscera of the body.
Sensory information from internal organs (namely, the blood vessels, the heart, and the
abdominopelvic organs) reaches the CNS levels of medulla, pons and hypothalamus, often
without ever reaching sensory cortex of your cerebrum and, thereby, not reaching
conscious awareness. These inputs elicit reflex responses through the efferent autonomic
nerves. As necessary, the ANS neurons elicit appropriate reactions of the heart, the
vascular system, and all the organs of the body in response to variations in the
environment – physical or biochemical (See the Homeostasis and Integrated Function
section for more about role of the autonomic nervous system in maintaining the
environment of the body).
The following table compares basic structural and functional features of the SNS and the
ANS:
| Comparison of the Somatic and the Autonomic Divisions of the Nervous
System. |
|---|
| Somatic |
Autonomic |
| Involves both afferent and efferent pathways |
Involves only the efferent pathway |
| Voluntary activities, including locomotion (effectors are skeletal muscles) |
Involuntary activities (effectors are cardiac muscle, smooth muscles, fat cells,
and glands) |
| Efferent signals originate at the cerebral cortex as a conscious decision and
activate neurons in the brainstem or spinal cord |
Unconscious signals originate in hypothalamus, brain stem, and spinal cord and
activate target neurons that lie in the peripheral nervous system |
| Brainstem and spinal cord neuron exerts direct control over skeletal muscle (One
neuron system, where there are no intermediate synapses between the CNS and the
target organs). |
Target neurons in the peripheral nervous system are grouped in ganglia and their
axons exert direct control over smooth muscle, cardiac muscle, glands, and fat
cells. (Two neuron system, where the efferent neurons synapse once outside the
CNS before the signals reach the target organs.) |
| Efferent axons are myelinated (fast conduction) |
Postsynaptic axons are non-myelinated (slow conduction); presynaptic axons are
myelinated. |
| Neuromuscular junctions are specific and localized |
Synapses at the target organs for the axons of the ANS may be diffuse
(varicosities). |
| Target organs (skeletal muscles) are always stimulated into action. |
ANS used several different neurotransmitter molecules (predominantly ACh and
norepinephrine, NE) |
|
The two parts of the autonomic nervous system are organized differently. The
parasympathetic nervous system is derived from preganglionic neurons in the brainstem
and from preganglionic neurons in the lateral horn of the spinal cord at sacral levels.
Preganglionic neurons of the sympathetic nervous system lie in the lateral horn of the
spinal cord at thoracic and lumbar levels of the spinal cord. This differential
distribution of the preganglionic neurons of the two systems gives rise to the names
“craniosacral division” for the parasympathetic nervous system and “thoracolumbar
division” for the sympathetic division. This is depicted in a generalized manner and will be discussed in detail below.
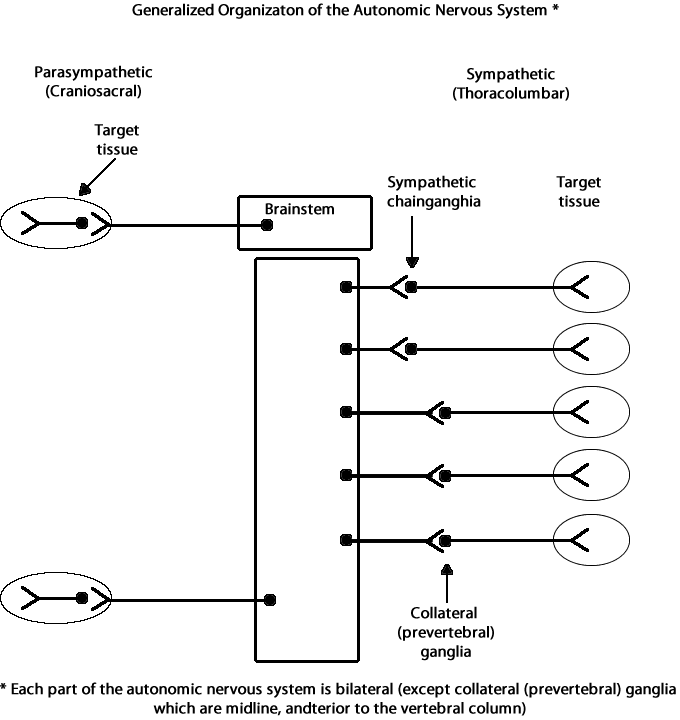 Image drawn by Debra McLaughlin.
Image drawn by Debra McLaughlin.
Parasympathetic preganglionic neurons originate from midbrain, pons, medulla oblongata,
and lateral horn of the spinal cord at sacral levels S2-S4. Axons of parasympathetic
preganglionic neurons travel to synapse at or within their target tissue.
Sympathetic preganglionic neurons originate from the lateral horn of spinal cord segment
levels thoracic T1 -T12 and lumbar L1-L2.
At the cervical and upper thoracic levels, axons of preganglionic sympathetic neurons
synapse onto postganglionic neurons that originate at the sympathetic chain ganglia
which lies to the side of the spinal cord and vertebral column, i.e., paravertebral. At
spinal cord levels T1-L2, axons of preganglionic neurons pass through the sympathetic
chain and synapse onto postganglionic neurons in one of the three midline collateral
(prevertebral, i.e., lying anterior to the vertebral column) ganglia.
Postganglionic neurons release neurotransmitter onto the target tissue, resulting in
either excitation or inhibition of cells in the target gland, muscle, or tissue.
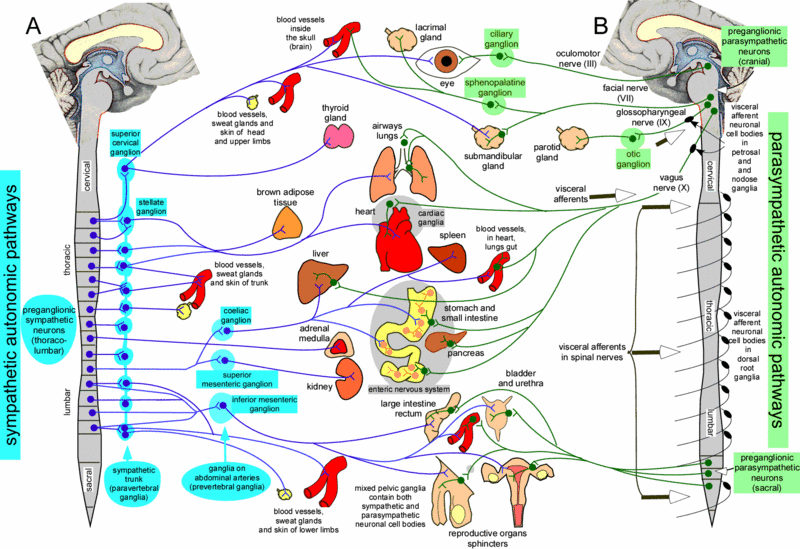 http://www.scholarpedia.org/article/File:Autonomic_nervous_system_main_figure_Blessing.gif
http://www.scholarpedia.org/article/File:Autonomic_nervous_system_main_figure_Blessing.gif
The cell bodies of the preganglionic neurons in the sympathetic division lie in the lateral horns of the
spinal cord from the segments T1 through L2. The axon of the preganglionic neuron typically exits at
the same level to synapse with the cell bodies and dendrites of the postsynaptic sympathetic neurons.
These postsynaptic neuronal cell bodies make up the paravertebral ganglia that lie close to the spinal
cord (around- or by the vertebrae) or sympathetic chain ganglia (the ganglia form a chain like structure
on either side of the vertebral column). As described below, the sympathetic chain has ganglia adjacent
to the thoracolumbar vertebrae, as well as adjacent to some cranial and sacral vertebrae.
Adjacent paravertebral ganglia are connected via ascending and descending preganglionic sympathetic fibers
forming longitudinal cords running parallel to the spinal cord. These connecting branches may be referred to
as interganglionic rami (ramus = branch). Together with the ganglia, they form the sympathetic trunk on either
side (bilateral) of the vertebral column. Its cephalic end continues into the skull through the carotid canal,
while caudally it converges with its counterpart anterior to the coccyx.
As mentioned in a previous comparison table (row 4), the ANS has a sequential two-neuron efferent pathway,
where each autonomic ganglion contains the synapse between the preganglionic neuron (the neuron that exits the CNS)
and the postganglionic neuron (the neuron that innervates the target organ).
The presynaptic sympathetic fibers entering the sympathetic chain may:
 Three possibilities of what happens when presynaptic sympathetic fibers enter the sympathetic chain. Drawn by Debra McLaughlin. Three possibilities of what happens when presynaptic sympathetic fibers enter the sympathetic chain. Drawn by Debra McLaughlin.
|
- synapse immediately on the postsynaptic neuron in the ganglion located at the same level it entered;
- ascend or descend down the sympathetic trunk before synapsing in a ganglion located at a different level;
- pass through the sympathetic chain ganglia without synapsing at all, synapsing instead in a prevertebral
ganglion (also known as the collateral ganglion).
|
As you will see shortly, the internal organs of the abdomen
and pelvis are primarily supplied by the fibers of this third pathway, while, generally, structures located
in the head, neck, body wall, limbs and thoracic cavity are innervated by one the first two pathways.
The nerve fibers that constitute the parasympathetic division originate at
the two anatomical ends of the central nervous system. The cranial nerves, CN III, CN
VII, CN IX and CN X and the sacral spinal nerves S2, S3 and S4 carry the presynaptic
(or, pre-ganglionic) parasympathetic outflow. The presynaptic neurons of the sacral part
of the Craniosacral division lie in the lateral horns of the spinal cord at the
appropriate level. These neurons extend into the body’s internal organs only and synapse
at the terminal (that lie close to the organ) or intramural
(within the target organ) ganglia. The exceptions are the four paired parasympathetic
ganglia of the head and neck. Of the four cranial nerves mentioned above, CNIII
supplies the ciliary ganglion; CNVII supplies the
pterygopalatine and submandibular ganglia; and
CNIX supplies the otic ganglion. The Vagus nerve
(CNX), as you have learned before, gets its name from its “wandering” nature.
It does wander in the thoracic and abdominopelvic cavities in your body to synapse on
postsynaptic (or postganglionic) neurons that innervate the heart, bronchi, stomach,
liver and the intestines. There are no named ganglia for these organs.
As described earlier, sympathetic preganglionic neurons originate from the
lateral horn of the spinal cord at levels T1-L2.
However, sympathetic innervation impacts on all levels of the body and
includes distributions to cervical as well as sacral regions of the body.
The cervical and stellate ganglia of the sympathetic chain contain the
postganglionic neurons that will innervate skin and organs of the head,
neck, and upper torso.
Sympathetic Ganglia Organization: Cervical Level
| Postganglionic Neuron Group |
Postganglionic Neuron Innervation Target Organ, Gland, or Tissue |
| Superior Cervical Ganglion |
pupil of eye, salivary glands, heart |
| Middle Cervical Ganglion |
heart, lungs, bronchi |
| Inferior Cervical Ganglion and Stellate ganglion (upper thoracic
level contribution); together these two ganglia are referred to as the
cervicothoracic ganglion |
Heart, lungs, arms, lower cervical neck, cranial arteries, sweat gland, blood vessels |
Abdominal peritoneal organs are
innervated by branches of postganglionic neurons that receive input from
lateral horn preganglionic neurons at the thoracic and lumbar levels.
Sympathetic Ganglia Organization: Thoracic and Lumbar Levels
| Greater splanchnic (T5-T9) |
Celiac |
Stomach, liver, gall bladder, bile ducts, pancreas, adrenal medulla chromaffin cells, kidney |
| Lesser (Middle) splanchnic (T9-T12) |
Superior mesenteric (aortico-renal) |
Small intestines, ascending colon, transverse colon |
| Least (Inferior) Splanchnic (T11--L2) |
Inferior mesenteric (renal) |
kidney |
| Pelvic splanchnic (L1-L5)
Sacral level |
Superior mesenteric
Inferior mesenteric |
Bladder, prostate, external genitalia, colon, rectum, uterine muscles, ovaries, testes
bladder, prostate, external genitalia, uterine muscles, ovaries, testes, pelvic viscera |
Organization of Parasympathetic Nervous System Ganglia
The table below provides a tabulated view of the local distribution of the two unique sources
of parasympathetic ganglion preganglionic neurons. Note that preganglionic neurons from brainstem cell
groups provide the efferent sources for small intestines, liver, stomach, and all organs in the
torso and abdomen that are superior to the intestines.
| Preganglionic Neurons |
Postganglionic Neuron Location |
Innervation Target |
| Cranial Brainstem Level |
| Edinger-Westphal nucleus of oculomotor complex |
Ciliary ganglion |
Ciliary muscle to adjust pupil |
| Lacrimal nucleus |
Pterygopalatine ganglion |
Lacrimal and nasal glands |
| Superior salivatory nucleus |
Submandibular ganglion |
Submandibular and sublingual glands |
| Inferior salivatory nucleus |
Otic ganglion |
Parotid gland |
| Nucleus ambiguus |
Un-named, at or within target |
Heart |
| Dorsal nucleus of the vagus nerve |
Un-named, at or within target |
Bronchi, stomach, liver, intestines |
| Sacral Level |
| Lateral horn of spinal cord at S2-S4 level |
Un-named, at or within target |
Descending and sigmoid colon, rectum, bladder, prostate, external genitalia, uterine muscles, ovaries, testes, pelvic viscera |
 ANS Pathways. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/)
ANS Pathways. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/)
When we are faced with a situation that demands our earnest attention –
that is, whether to “fight or flee,” our brain needs to receive as much
oxygen as it can get (for processing sensory information); our respiratory
passageways need to open up for moving as much air as possible; and the
heart needs to pump more blood to move oxygen to the needed organs (brain
and skeletal muscles). When we are faced with such a situation, it is
unlikely that we will have time to eat (or even feel like eating), nor
will we have the time to waste on going to the bathroom; hence, these
functions will not be facilitated.
Within the autonomic nervous system, the sympathetic (“fight or flight”) and
the parasympathetic (“rest and digest”) functions may be viewed as
opposing each other. In general, the parasympathetic branch tends to
exert an inhibitory effect on the target cells, while the sympathetic
branch has an excitatory effect. Most organs, therefore, are innervated
by both these branches of the autonomic nervous system to facilitate
maintenance of homeostatic balance. As we learn more about these two
systems, their modes of action will be clearer. The following table
summarizes the major parasympathetic and/or sympathetic physiological
effects on target cells, glands, and muscles. Note that many organs are
dually innervated, while others are innervated only by the sympathetic
branch. Blood vessels of abdominal viscera, skin of the limbs, and
skeletal muscles are constricted by sympathetic nervous system activation;
blood vessels are minimally impacted by activation of the parasympathetic
nervous system except for certain areas of the body such as the blood
vessels of the face that are associated with blushing.
| Effects of the two branches of the ANS |
|---|
| Organ |
Sympathetic Effect |
Parasympathetic Effect |
| Pupil |
dilation |
constriction |
| Lens |
Far focus (lower curvature) |
Near focus (increased curvature) |
| Salivary Gland secretion |
High in viscosity |
serous |
| Heart |
Increased rate and pressure |
Lower rate and pressure |
| Lungs |
Dilation of respiratory passages |
Constriction of respiratory passages |
| Gastrointestinal |
Decreased motility |
Increased motility |
| Kidneys |
Decreased filtration rate |
Increased filtration rate |
| Male genitalia |
Ejaculation |
Erection |
| Vascular smooth muscle |
Variable depending on the neurotransmitter |
Relaxation |
| Sweat glands |
Increased activity |
No innervation |
| Arteries to skeletal muscle |
dilation |
No innervation |
| Veins |
Variable depending on the neurotransmitter |
No innervation |
|
Before we discuss the neurotransmitters for autonomic nervous system, let us refresh our memory about these
molecules. Neurotransmitters are chemical “signals” that travel from a neuron (presynaptic cell) to the
next cell (neuron, muscle, glands, etc.) to relay information necessary for the task that this “next”
cell should perform. Chemically, these molecules are quite varied, ranging from a simple gaseous molecule
(nitric oxide) to peptides (endorphin). The most widespread of all neurotransmitters in your body is
acetylcholine (ACh). This is the neurotransmitter we came across in an earlier chapter on skeletal
muscles. In the somatic nervous system, which is a “one neuron system” where the signal is carried by
one neuron from the CNS to the target organ, the neurotransmitter is always ACh which activates skeletal
muscles. In the parasympathetic division, this same neurotransmitter is released by both preganglionic
neuron (at the ganglionic synapse) as well as postganglionic neuron (at the effector synapse). In the
sympathetic division, the preganglionic neuron releases the same ACh as the neurotransmitter, but the
postganglionic neurons release norepinephrine (NE; a catecholamine synthesized from phenylalanine).
However, if the target organ for the postganglionic sympathetic neuron is a sweat gland, the
neurotransmitter is again ACh.
The longest of the preganglionic sympathetic fibers do not synapse in any conventional ganglia but directly onto
the chromaffin cells of the adrenal medullae. The chromaffin cells can be thought of as modified postsynaptic
neurons that form microganglia. These medullary cells, upon stimulation by ACh released by the sympathetic
preganglionic neuron, release epinephrine and, to a lesser extent, norepinephrine. These molecules function
as hormones of the sympathetic system, and are released into the bloodstream, which propagates the sympathetic
response throughout the body.
As noted previously, a balance of function in the autonomic nervous system is required to maintain a
living organism. Activity in the autonomic nervous system is not a conscious endeavor that most people
can normally control (however, with practice, autonomic nervous system can be controlled to some extent
such as in deep meditation). Sympathetic and parasympathetic functions that maintain homeostatic balance
and other internal body states are listed below.
Parasympathetic Functions:
- Stimulates visceral activity
- Decreases metabolic rate
- Decreases heart rate and blood pressure
- Conserves energy and promotes sedentary activities
- Increased salivary gland secretion (serous, and hence dilute)
- Increased digestive secretions
- Increased motility and blood flow in digestive tract
- Facilitation of urination and defecation.
Sympathetic Functions:
- Heightened mental alertness
- Increased metabolic rate
- Increased respiratory rate and dilation of respiratory passageways.
- Viscous saliva secretion (higher protein content, less water)
- Increased heart rate and blood pressure
- Energy reserves activated
- Reduced digestive and urinary functions
- Sweat glands activated
As you might expect, certain organs and tissues receive innervation from both the sympathetic and parasympathetic
nervous systems. These organs are said to be “dually innervated.” An example of this is how the parasympathetic
division facilitates micturition and defecation, while sympathetic input activates sphincters of the bladder
and the rectum, impending micturition and defecation, respectively.
Example
Dually Innervated Heart and Lungs
The heart and lungs are dually innervated. Alterations of the pace and the force of contraction of heart muscles
are influenced by the effect of the vagus nerve on the pacemaker cells of the heart. Parasympathetic input to
both right and left vagus (CN X) nerves provide cervical cardiac nerves to the cardiac plexus. The preganglionic
sympathetic fibers to the heart originate from the T1-T4/ T5 of the spinal cord. Normal heart rhythm is in the
60-100 beats per minute range. During restful conditions, the vagus nerve (CN X), through parasympathetic input,
has the dominant influence that maintains the heart rate in the 60-80 beats per min (bpm) range. Under stress,
excitement, or exertion, sympathetic stimulation can shift the heart rate to greater than 100 bpm. A slower than
normal heart rate, less than 60 bpm, mediated by vagus nerve and the parasympathetic nervous system or an illness,
is referred to as bradycardia. A faster than normal heart rate can be mediated by sympathetic nervous systems or
can be due to illness, and is referred to as tachycardia.
For lung innervation, the sympathetic and parasympathetic divisions of the autonomic nervous system nerves are
arranged as the pulmonary plexi and innervate the smooth muscle and glands of the bronchi and pulmonary blood
vessels. The sympathetic preganglionic fibers to the lungs arise from T1-T4. The sympathetic stimulation of
the bronchial smooth muscles is achieved by the epinephrine released from adrenal glands. Overall, the
sympathetic stimulation causes vasoconstriction and bronchodilation in the lungs. Stimulation by
parasympathetic input causes vasodilation and bronchoconstriction in the lungs.
 Dually innervated cardiac nerves. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Dually innervated cardiac nerves. This work by Cenveo is licensed under a Creative
Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).Everybody
is familiar with asthma. Whatever the causality, asthma is
characterized by bronchoconstriction due to increased sensitivity to
irritating stimuli (which could be due to allergic response to certain
antigens or due to irritants in the environment like coal dust, exhaust
fumes, etc.). Increased mucus secretions rapid bronchoconstriction
leads to labored breathing which we know as “asthma.”
There are certain effectors in your body that are not dually innervated.
Sweat glands, arrector pili muscles, adrenal medula, liver, adipocytes,
lacrymal glands, radial muscle of the iris, juxtaglomerular apparatus,
uterus and most vascular smooth muscles have only sympathetic
innervation. In contrast to the sympathetic system, there are
relatively few organs that function only with parasympathetic
stimulation. Examples of such organs are the circular muscle of iris
which causes pupillary constriction and the parietal cells of the
stomach that secrete gastric acid.
Most vasculature of smooth muscles receive only sympathetic innervations.
The balance of vascular constriction and dilation is mediated by
sympathetic tone. The tone of a single vessel is proportional to the
sympathetic stimulation it is receiving. More stimulation leads to
more constriction, and, as sympathetic input to the vessel decreases,
the vessel relaxes. Also, it is important to recognize that the impact
of the release of epinephrine or norepinephrine on blood vessels is
dependent on the subtype of neurotransmitter receptor on those blood
vessels. For example, sympathetic activation leads to constriction of
most vasculature in the body (mediated through stimulation of
alpha-type receptors), but leads to dilation of coronary vessels
supplying the heart (mediated through stimulation of beta-type
receptors). The difference in how the vessel responds is due to
the type of adrenergic receptor, and, ultimately, what type of
intracellular signaling pathways are engaged to drive the vascular
response. Adrenergic receptors are stimulated by either
norepinephrine or epinephrine.
Most vessels in the body have the alpha-type receptor for binding
norepinephrine, which results in vasoconstriction. Vasculature of
the heart, on the other hand, has beta-type receptors for binding
norepinephrine, which results in vasodilation. Also, recall that
sympathetic nervous system activation causes release of epinephrine
by chromaffin cells of adrenal medullae. Epinephrine has a greater
effect on stimulating beta receptors than does norepinephrine, which
means that epinephrine has a stronger effect on cardiac stimulation
and a much weaker effect on blood vessels in muscles. Stimulation of
beta receptors in the heart causes dilation of coronary blood vessels supplying the
heart, increase heart rate, and increase in the strength of contraction
of cardiac muscle. Blood vessels in skeletal muscles express alpha
and beta adrenergic receptors. During exercise, blood vessels in
skeletal muscle dilate, and, among many possible factors (ATP, lactic
acid, carbon dioxide, oxygen, adenosine) that could cause this
vasodilation, circulating epinephrine released into the bloodstream
by chromaffin cells in the adrenal medullae is one other possible
factor.
Before taking the quiz below, consider again the learning objectives for this unit. Could
you demonstrate each of these objectives? If so, you will be ready for the assessment
below. If not, consider reviewing content related to these objectives before attempting
the assessment.
- Classify the organs that are part of the nervous system as belonging
to the central nervous system (CNS) or the peripheral nervous system
(PNS).
- Within a neuron, identify the soma, axon and dendrite and describe the main function of each region.
- Identify neurons based on anatomical features: unipolar, bipolar,
multipolar and anaxonic and based on functional properties: sensory,
motor, interneuron.
- List the four types of CNS glial cells and describe their function.
- List the two types of PNS glial cells and describe their function.
Describe the anatomical relationship between the glial cells and the
PNS.
- Compare the structure of myelinated vs. unmyelinated axons. Distinguish between white matter and gray matter.
- Describe the transmembrane potential or voltage across the cell membrane and how it is measured.
- Contrast the relative concentrations of ions in body solutions
inside and outside of a cell (sodium, potassium, calcium and chloride
ions).
- Explain how four factors determine a neuron’s resting membrane potential.
- Explain how a local electrical response in a neuron membrane is caused by stimulation.
- Interpret a graph showing the voltage vs. time relationship of an action potential.
- Explain action potential.
- Identify the presynaptic and postsynaptic cells at a synapse.
- Explain synaptic transmission in terms of the structural and functional features of electrical and chemical synapses.
- Explain how a single neurotransmitter may have different effects at different postsynaptic cells.
- Explain temporal and spatial summation of synaptic potentials and discuss how action potentials differ from synaptic potentials.
- Identify the four classes of neurotransmitters and identify the most common excitatory and inhibitory neurotransmitters.
- Explain the role of the autonomic nervous system as a motor division of the nervous system.
- Compare the somatic and autonomic nervous systems.
- Contrast the anatomy of the parasympathetic and sympathetic systems.
- Describe the local organization of each of the sympathetic and
parasympathetic systems, including the pattern of innervation of target
glands, organs, and tissues.
- Describe major parasympathetic and sympathetic physiological effects on target organs.
- Identify the neurotransmitters released by preganglionic and
postganglionic neurons in the sympathetic and parasympathetic nervous
systems and describe their effects.
- Describe examples of specific effectors dually innervated by the
autonomic nervous system and explain how each branch influences function
in a given effector.
- Name examples of effectors innervated either by only the sympathetic
branch or by only the parasympathetic branch of the autonomic nervous
system and explain how that branch by itself influences function in a
given effector.
Recall that the nervous system often plays important roles in homeostasis, including our
ability to respond to both our internal and external environment. In order to respond
and maintain homeostasis, we must be able to detect that a change has happened in the
first place. The nervous system is well adapted to carry out this specific function, by
converting various internal and external stimuli into electrical signals in the form of
post-synaptic potentials or action potentials. Any action potentials generated are then
carried along neurons, and various forms of electrical signals at synapses and neural
networks are integrated for decision making purposes. Not all stimuli are used only for
homeostasic maintenance, with sensory input playing a role in all kinds of reflex and
conscious responses as well.
In this section we will look more closely at how the nervous system converts physical and
chemical signals such as touch, taste, light, sound, and others into electrical signals
that can be interpreted and processed by the central nervous system in a manner that
allows us to perceive them. This overall process of sensing and interpreting these
signals is called sensation. For the purposes of study, sensation is typically divided
into general and special senses. Regardless of whether it is a general or special sense,
the signal is received by receptors that then convey information to the CNS.
Sensory receptors enable us to learn about the environment around us or about the state
of our internal environment. Stimuli from varying sources, and of different types, must
be received and changed into the electrochemical signals of the nervous system
represented by changes in the membrane potential. The sensory information is relayed to
the central nervous system where it is integrated with other sensory information, or
sometimes higher cognitive functions, to become a conscious perception of that stimulus.
The central integration may then lead to a motor response.
Sensory Receptors
Stimuli in the environment activate specialized receptors in the peripheral
nervous system. The classification of receptors into types can be based on three
different criteria: structure of the receptors, location of the receptors
relative to the stimuli they sense, and by the types of stimuli to which they
respond. Regardless of type, the function of these receptors is to transduce a stimulus from one form of energy (chemical,
physical, etc.) into a change in the cell membrane potential that may or may not
create an action potential.
Structural Receptor Types
The cells that detect a change in the environment can be neurons with free nerve endings, where the dendrites are exposed to the
surrounding tissue; neurons with encapsulated endings,
where supporting cells aid in the reception of stimuli; or
specialized receptor cells, which have specific structural components
for detecting stimuli. Examples of neurons with free nerve endings
are the pain
and temperature receptors in the dermis of the skin. Also in the
dermis are
encapsulated nerve endings such as the lamellar corpuscle that
senses pressure.
The cells in the retina that receive light stimuli are an example
of specialized
photoreceptor cells, not neurons, that in turn can
stimulate an associated sensory neuron.
 Structural Receptor Classification. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Structural Receptor Classification. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Receptors can also be classified based on their location relative to the stimuli.
Exteroceptors are receptors that receive input from
the external environment, such as the lameller corpuscles of the dermis and
photoreceptors of the eye that have already been mentioned. Interoceptors are those that sense stimuli from the internal organs.
Examples would include a stretch receptor in the wall of an organ, such as those
that sense the increase in blood pressure in the aorta or carotid artery or
detects stretch as the bladder fills with urine. Finally, proprioceptors are widely distributed receptors in muscles, tendons
and joint capsules that the body uses to determine position and movement of
structures, such as its limbs and fingers. Proprioceptors allow you to touch
your finger to your nose, even with your eyes closed.
Functional Receptor Types
Lastly, receptors can be classified by the types of signals they transduce into
changes in membrane potential.
Chemoreceptors sense chemical stimuli, examples being
taste, smell and the osmotic pressure of the body’s extracellular fluids (the
latter sensed by osmoreceptors).
Nociceptors are pain receptors. Although pain is primarily
a chemical sense that detects the presence of chemicals released during tissue
damage, nociceptors are typically considered in a functional category of their
own. Nociceptors are found in most tissues throughout the body, exceptions being
the brain and possibly certain internal structures of organs.
Mechanoreceptors sense physical stimuli, such as pressure
and vibration, as well as the sensation of sound and pull of gravity. A specific
example of a mechanoreceptor is the baroreceptors (pressure receptors) found in
the carotid arteries, which sense blood pressure.
Thermoreceptors are specific to sensing temperature and
changes in temperature. Thermoreceptors are found in two forms, those that
respond most strongly to temperatures below normal body temperature (cold
thermoreceptors), and those that respond most strongly at temperatures above
normal body temperature (warm thermoreceptors). At normal body temperature, both
types of receptors are active, but there is generally no awareness of cold or
warmth.
Photoreceptors respond to electromagnetic radiation
(light). Humans have the ability to sense electromagnetic waves at wavelengths
between 400 and 700 nanometers, with different wavelengths corresponding to
different colors.
Because the central nervous system requires a significant amount of input in order to
carry out homeostatic functions, receptors are numerous. Some of these receptors are
widely distributed throughout body tissues (general or somesthetic
senses), while others are localized to special sense organs of the head, such
as the eye or ear (special senses). Collectively, the receptors
and associated neurons that sense and process information related to our somesthetic
senses are called the somatosensory system. We will first review
the general categories of receptors found in this system, along with their
distribution.
 Anatomical divisions of the general senses. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomical divisions of the general senses. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The widely distributed receptors of the somesthetic senses can be classified based on the
anatomical structures in which they are located. They are termed somatic senses when they are located in, and sense information from, the
somatic structures of skin, muscles, joints (including the related structures of tendons
and ligaments) and organ capsules. The somesthetic senses also include visceral senses, the ability to sense the chemical environment of
our blood and body fluids. Visceral senses also include monitoring the “state” of
internal organs (ex. pressure and stretch receptors in vessels and in organs other than
skin, muscles and joints). Visceral senses are particularly important to the
function of the autonomic nervous system, whose function is to maintain many of these
variables near a set point.
 Sensory receptors vary in distribution and anatomical types in
the skin. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Sensory receptors vary in distribution and anatomical types in
the skin. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Within an organ, receptors will be found in various places. For example, in the skin,
there are sensory receptors in both the epidermis and dermis; and in skeletal
muscles, sensory receptors are found embedded in the contractile elements as well as in
the tendons.
The types of receptors (by structural and functional classifications) found in an organ
also vary. The variety of receptors that we have contributes to our ability to sense
multiple modalities (types of sensations), and sub-modalities, as described in the next
section. Receptors of specific modalities have their own distributions, depending on
need; nociceptors are found in most tissues, while proprioceptors are limited to muscles
and joints and their related supporting structures. A summary of specific receptor
types, locations and stimuli are shown in table below. The anatomy of some of these
receptors is shown in figure above. The topic of sensory adaptation in the last column of
the table will be further discussed in the section on sensory adaptation.
| Specific Receptors of the Somatic Sensory System |
|---|
| Name |
Historical name |
Location(s) |
Stimuli |
Adaptation |
| Free nerve endings |
* |
Dermis, cornea, tongue, joint capsules, visceral organs |
Pain, temperature, mechanical deformation |
Depends on specific type |
| Type I mechanoreceptors |
Merkel discs |
Epidermal-dermal junction, mucosal membranes |
Low frequency vibration (5 – 15 Hz) |
Slow |
| Bulbous corpuscle |
Ruffini corpuscle |
Dermis, joint capsules |
Stretch |
Slow |
| Tactile corpuscles |
Meissner corpuscle |
Papillary dermis, especially in the fingertips and lips |
Light touch, vibrations below 50 Hz |
Rapid |
| Lamellar corpuscle |
Pacinian corpuscle |
Deep dermis, subcutaneous tissue |
Deep pressure, high-frequency vibration (around 250 Hz) |
Rapid |
| Hair follicle plexus |
* |
Wrapped around hair follicles in the dermis |
Movement of hair |
Rapid |
| Muscle spindle |
* |
In line with skeletal muscle fibers |
Muscle contraction and stretch |
Rapid |
| Tendon stretch organ |
Golgi Tendon organ |
In line with tendons |
Stretch of tendons |
Slow |
|
*no corresponding historical name
The receptors that provide information for somesthetic senses come in a variety
of anatomical and functional types. Each specific receptor will respond to only
one type of functional signal (such as mechanical or chemical, but not both). If
the information is transmitted all the way to the brain’s cortex, we perceive a
sensation. The type of perception that this information leads to is called a modality. There are four main modalities typically
recognized as part of the somesthetic senses. These include temperature, touch,
pain (nociception), and position and movement (proprioception). Yet each of these can be further
subdivided into sub-modalities (sometimes called stimulus
modalities). For example, the modality of pain can be subdivided into
sharp, dull and aching.
There is not general agreement over how sensory modalities should be categorized
and subcategorized. In some classification schemes the general sense of touch is
replaced with pressure. In others, there may be differences in the subcategories
of touch. One classification scheme is presented in the table below.
| One Scheme for Categorizing Sensory Modalities |
|---|
| In
subdivision 2, touch is a sensation produced under conditions of very little
skin displacement, while pressure requires displacement of the skin and
underlying tissues. Flutter is a sensation produced by a stimuli acting at a
lower frequency than vibration. |
| Main Modality |
Subdivision 1 |
Subdivision 2 |
| Touch |
Crude touch |
|
|
Discriminative touch |
Touch |
|
|
Pressure |
|
|
Vibration |
|
|
Flutter |
| Pain (Nociception) |
Sharp |
|
|
Dull |
|
|
Aching |
|
| Temperature |
Hot |
|
|
Cold |
|
| Proprioception |
Position |
Muscle length |
|
|
Muscle tension |
|
|
Joint pressure |
|
Movement |
Muscle length |
|
|
Muscle tension |
|
|
Joint pressure |
|
|
Joint angle |
|
The stimulus modalities shown in the subdivision 1 and subdivision 2 columns in
the table might contribute to more complex sensations when combined under
certain conditions. For example, there is evidence that a tickle occurs with
simultaneous activation of certain touch and pain receptors – as long as other
conditions are appropriate. As you are probably aware, others can tickle you,
but you generally cannot tickle yourself, even if you can activate the same
sensory receptors. Thus input from other systems seems to be able to affect our
sensations. There is still a lot for scientists to learn about sensory
perception.
Sensory Adaptation
When first jumping into cool water, you may endure a wave of sensory information
“reminding you” that the water is cool. Yet minutes later, you may be “used to” the
water temperature, or have adapted to it. This change in perception did not occur
because the water warmed up, but because the sensory receptors that originally responded
to the change in temperature are no longer sending signals to the CNS, or are sending
them at a decreased rate. This example indicates how a rapidly adapting receptor might
function; it provides significant signals to the CNS about the original change in
temperature that the body experiences, but then adapts such that it sends fewer signals
thereafter.
Not all sensory receptors are rapidly adapting. Certain pain receptors seem to have
little adaptation, or are very slowly adapting, so that as long as the pain stimulus is
applied, the person continues to “get the message”! The general rates of adaptation for
specific sensory receptors are indicated in the above table.
We have all felt pain, and although uncomfortable, it likely provided us important
information about tissue damage -- damage that may have gotten worse if pain had not
made us aware of the problems at hand. In response to pain we tend to “protect” the
damaged tissue from further use and seek appropriate medical attention. Thus pain is a
critical sensation for alerting us to problems within the body such that they can be
appropriately addressed.
Pain receptors, called nociceptors, are spread throughout
most of the body’s tissues, with the exception of the central nervous system.
They respond to nociceptive, or noxious, stimuli that lead to our perception of
pain. These receptors vary in the specific stimuli that they respond to, as well
as how quickly they transmit information to the central nervous system.
You likely realize that there are many noxious stimuli. Extreme temperature, a
pinch, blunt impact, cuts, intestinal gas, overuse of joints, and others can all
elicit the sensation of pain. This is because all of these stimuli have the
ability to either directly activate nociceptors, or cause tissue damage that
leads to the release of chemical substances that will activate nociceptors. You
also appreciate that the sensation of pain can change over time, where an injury
may start out with stinging pain, become dull, and even revert to stinging again
under certain conditions, such as when someone touches the injured area. The
combination of nociceptors stimulated helps determine the characteristics of the
pain that is felt. Examples of nociceptor locations and types are listed in
Table below.
| Types of Nociceptors |
|---|
| Location |
Nociceptor type |
Activated by: |
| Skin |
Mechano- |
Intense mechanical stimulation |
|
Chemo- |
Many chemical mediators released during tissue damage, including
prostaglandins and histamine |
|
Thermo- |
Mechanical and thermal stimuli |
|
Polymodal |
High intensity stimuli of various types |
|
Silent |
Mechanical stimulation after inflammation has set in |
| Viscera |
Mechano- |
Intense mechanical stimulation |
|
Thermo- |
Mechanical and thermal stimuli |
|
Chemo- |
Many chemical mediators released during tissue damage, including
prostaglandins and histamine |
|
Silent |
Mechanical stimulation after inflammation has set in |
| Joints |
Mechano- |
High intensity mechanical stimulation |
|
Polymodal |
Various |
|
Silent |
Mechanical stimulation after inflammation has set in |
|
The silent (sleep) nociceptors listed in the table have the unique property that
they are normally unresponsive to stimulation until turned “on” by chemicals
released during the inflammatory process. This is one reason why, after you have
stubbed your toe, or pinched your finger, you may have thought to yourself “this
is going to hurt”. You know that once the tissue inflames (swells up), throbbing
pain is likely to set in as further nociceptors become activated.
Besides the noxious stimulus that activates a receptor, the type of axon (fiber)
that the receptor neuron contains also contributes to how we perceive pain. In
general, fibers can be divided into 2 categories, with the properties listed in
table below.
| Fiber type |
Features |
| A-delta |
2-5 mm diameter; Myelinated with fast conduction velocities; Small receptive
fields for precise localization of pain; Mainly transmit from mechanical
and thermal nociceptors |
| C fibers |
0.4-1.2 mm diameter; Unmyelinated with slow conduction velocities; Large
receptive fields with less precise localization of pain; Carry signals
from many types of nociceptors |
Categories of Pain
Because there are multiple types of nociceptors that can transmit information
at different rates, our pain sensation is not always the same. Scientists
generally recognize three different pain categories (sensations or stimulus modalities),
as described in table below.
| Categories of Pain |
|---|
| Pain Category |
Description |
Fiber type |
| Fast pain/pricking pain/sensory pain |
Sharp, stinging pain that is well localized. Arises mainly from the skin. |
A-delta |
| Burning pain/ soreness pain |
Elicited by inflammation secondary to damaged tissue (hit thumb with hammer,
etc.). Arises mainly from skin, but also from tissues such as muscle. It is more
diffuse and longer in duration than fast pain. |
C fibers |
| Aching pain |
Poorly localized pain arising from deep structures (joints and viscera). |
C fibers |
|
Hyperalgesia and Analgesia
Notice that because chemical and silent nociceptors are activated after tissue
injury has set in, our sensations of pain can change over time. An initial cut
will activate mechano-nociceptors, sending the fast pain signals along A-delta
fibers to the brain. After inflammation in the area has set in, the chemical
and/or silent nociceptors may send information along C fibers, producing a
different pain sensation. If these silent nociceptors become sensitized by the
inflammatory mediators, then we might experience hyperalgesia, where tissue stretch to an injured area can be sensed as
a more intense pain.
Our ability to provide analgesia (reduce or block pain) is
usually directed at inhibiting the local formation of the mediators that
activate chemical nociceptors (this is how aspirin and ibuprofen work), blocking
the transmission of signals along peripheral pain fibers (local anesthetics), or
interrupting the transmission of pain signals in the CNS (endogenous or
exogenous opioids as well as general anesthesia). You may have had experience
with a local anesthetic during dental work or when stitches were put in. They
act by blocking the ability of nerve fibers to conduct action potentials. Local
anesthetics block C fibers more easily, but in time the A-delta fibers are also
blocked. This is why the dentist waits awhile after giving the local anesthetic
before commencing the work that would otherwise cause pain – you want to make
sure your A-delta fibers are fully blocked as well!
CNS Role in Pain
Although the focus of this section has been on the peripheral sensation and
transmission of resulting action potentials, keep in mind that the processing of
pain, such as pain sensation, localization and the physical and emotional
responses to pain, are functions of the CNS.
Receptive Field
Although the receptors of the somesthetic senses are widely distributed throughout the
body, the density of receptors varies from place to place. This often leads to
corresponding changes to the sizes of the receptive field for any
modality. The receptive field is the area from which a sensory receptor, and its
corresponding neuron, can detect a stimulus.
 Receptive fields. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 Unitied States (http://creativecommmons.org/licenses/by/3.0/us/).
Receptive fields. This work by Cenveo is licensed under a Creative Commons Attribution 3.0 Unitied States (http://creativecommmons.org/licenses/by/3.0/us/).
The size of a receptive field might vary from a few square millimeters to tens of square
millimeters, depending on receptor type and location. With larger receptive fields, the
CNS has less ability to localize stimuli or to differentiate between multiple stimuli.
This can be demonstrated by a two-point discrimination test where two thin objects (such
as pen tips) are touched to a person’s skin in proximity to one another. If this is done
on the upper back, even objects that are a centimeter apart may be interpreted by the
individual to be a single object. The same distance between objects on the lips or
fingertips is easily interpreted by the CNS as separate objects. Receptor densities and
the corresponding size of receptive fields are represented in the CNS as the sensory
homunculus with the primary somatosensory cortex.
 Sensory homunculus. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Sensory homunculus. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Individual sensory neurons that are in proximity to each other are bundled
together into nerves that enter the spinal cord through its posterior root or
horn. The region of the skin that each spinal nerve carries information from is
called its dermatome. Because there are 31 spinal nerves, there are an equal
number of dermatomes, each named for the spinal nerve to which it sends
information.
 Dermatomes. By Mikael Haggstrom (en.wikipedia.org/wiki/Dermatome_(anatomy) Public Domain.
Dermatomes. By Mikael Haggstrom (en.wikipedia.org/wiki/Dermatome_(anatomy) Public Domain.
A dermatome map is particularly useful in the diagnosis of spinal cord injuries
that interrupt spinal cord transmission. An injured person will lose sensation
from all their dermatomes below the level of injury.
Stretch Reflex
One of the simplest reflexes is a stretch reflex. In
this reflex, when a skeletal muscle is stretched, a muscle spindle in the belly of the
muscle is activated. The axon from this receptor travels to the spinal cord where it
synapses with the motor neuron controlling the muscle, stimulating it to contract. This
is a rapid, monosynaptic, ipsilateral reflex that helps to maintain the length of
muscles and contributes to joint stabilization. A common example of this reflex is the knee
jerk reflex that is elicited by a rubber hammer striking against the patellar tendon,
such as during a physical exam. When the hammer strikes, it stretches the tendon, which
pulls on the quadriceps femoris muscle. Because bones and tendons do not typically pull
muscles, the muscle “thinks” it is stretching very rapidly, and the reflex acts to
counteract this stretch. In doing so, the “knee jerk” occurs.
Along with the monosynaptic activation of the alpha motor neuron, this reflex also
includes the activation of an interneuron that inhibits the alpha motor neuron of the
antagonistic muscle. This aspect of the reflex ensures that contraction of
the agonist muscle occurs unopposed.
 Stretch Reflex. When a muscle is stretched (1), muscle
spindles (2) send information to the spinal cord (3) where it synapses on motor neuron
of the same muscle (4) causing it to contract (5). At the same time, stimulation of an
inhibitory interneuron (6) prevents contraction of the antagonistic muscle (7 and
8). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Stretch Reflex. When a muscle is stretched (1), muscle
spindles (2) send information to the spinal cord (3) where it synapses on motor neuron
of the same muscle (4) causing it to contract (5). At the same time, stimulation of an
inhibitory interneuron (6) prevents contraction of the antagonistic muscle (7 and
8). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Flexor (Withdrawal) Reflex
Recall from the beginning of this unit that when you touch a hot stove, you reflexively
pull your hand away. Sensory receptors in the skin sense extreme temperature
and the early signs of tissue damage. To avoid further damage, information travels along
the sensory fibers from the skin and into the posterior (dorsal) horn of the spinal
cord. Once in the spinal cord, the sensory fibers synapse with a variety of interneurons
that mediate the responses of the reflex. These responses included a strong initial
withdrawal of the flexor muscle (caused by activation of the alpha motor neurons),
inhibition of the extensor muscle (mediated through inhibitory interneurons), and
sustained contraction of the flexor (mediated by a spinal cord neuronal circuit). And as
already discussed, the sensory information will also travel to the brain to develop a
conscious awareness of the situation such that conscious decision-making can take over
immediately after the reflex occurs.
Crossed-Extensor Reflex
Imagine what would happen if, when you stepped on a sharp object, it elicited a strong
withdrawal reflex of your leg. You would likely topple over. In order to prevent this
from happening, as the flexor (withdrawal) reflex involving the injured leg happens, an
extension reflex of the opposite (contralateral) leg occurs at the same time, creating a
crossed-extensor reflex. In this case, the ipsilateral limb reacts with a
withdrawal reflex (stimulating flexor muscles and inhibiting extensor muscles on same
side), but the contralateral extensor muscles contract so that the person can
appropriately shift balance to the opposite foot during the reflex.
 Crossed-Extensor Reflex. In this reflex, as withdrawal
from the damaging stimulus occurs in the ipsilateral leg, extension occurs in the
contralateral leg as a way of maintaining balance. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Crossed-Extensor Reflex. In this reflex, as withdrawal
from the damaging stimulus occurs in the ipsilateral leg, extension occurs in the
contralateral leg as a way of maintaining balance. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The eyes are located within the skull orbits, which provide protection for the eyes, as
well as provide a place to anchor the soft tissues that support the functions of the
eye. The eyelids, with lashes at their leading edges, help to protect the eye from
abrasions by blocking particles that may get onto its surface. From the inner surface of
each lid, a thin mucous membrane known as the conjunctiva folds in and
covers the surface of the eye. Tears are produced by the lacrimal
glands, which are superior and lateral to the orbit in each eye, and they flow over the
conjunctiva to wash away particles that may have gotten past the lashes and the lids.
Tears flow down through the lacrimal ducts, located on the medial
side of each orbit, into the nasal cavity.
 Anatomical features of the tissues surrounding the eye (a) and
lacrimal system (b). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomical features of the tissues surrounding the eye (a) and
lacrimal system (b). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Components of the Eye
The eye itself is a hollow sphere composed of three layers of tissue. The outermost layer
is the fibrous tunic which is the white sclera
and clear cornea. The two parts of the fibrous tunic are
continuous, though they have different properties. The sclera accounts for 5/6 of the
surface of the eye, most of which is not visible (though humans are unique in having so
much of the “white of the eye” visible). The cornea covers the anterior region of the
eye and allows light to pass into the eye where it will eventually stimulate
photoreceptors. The next layer of the eye is the vascular tunic,
which is mostly composed of the choroid, a highly
vascularized connective tissue that provides a blood supply to the adjacent tissue. The
choroid is posterior to the ciliary body, a muscular structure
that is attached to the lens by the suspensory
ligament. The ciliary body focuses light on the back of the eye. Overlaying the
ciliary body, and visible in the anterior eye, is the iris, the
colored part of the eye that opens in the center as the pupil. The
innermost layer of the eye is the neural tunic, which is the retina or the nervous tissue that is responsible for
photoreception.
 Anatomical features of the eye. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomical features of the eye. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Chambers of the Eye
The eye is also divided into two cavities, the anterior and
posterior. The anterior chamber, of anterior cavity, is the space
between the
cornea and iris. The posterior chamber sits between the iris and
the lens. Both
the anterior and posterior chambers are filled with a watery fluid
called the
aqueous humor. The posterior vitreous
chamber (also posterior cavity) is posterior to the lens and is filled
with a more viscous fluid called the vitreous humor (vitreous body).
Movement of the eye within the orbit is accomplished by the contraction of six extraocular muscles that originate from the bones of the orbit and
insert into the surface of the eye.
 Muscles that Control Eye Movement. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Muscles that Control Eye Movement. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Each of these muscles is innervated by
one of the cranial nerves as summarized in the table below.
| Muscles that Control Eye Movement |
|---|
| Muscle |
Effect of Contraction |
Innervated by (including cranial nerve
number): |
| Superior rectus |
Eye rotates to look up |
Oculomotor nerve(III) |
| Medial rectus |
Eye rotates to look medially |
Oculomotor (III) |
| Inferior rectus |
Eye rotates to look down |
Oculomotor (III) |
| Lateral rectus |
Eye rotates to look laterally |
Abducens (VI) |
| Superior oblique |
Medial rotation |
Trochlear (IV) |
| Inferior oblique |
Lateral rotation |
Oculomotor (III) |
|
The retina, where the photoreceptors are found, is located at the posterior aspect of the
eye. In order for the retina to transmit the most appropriate information to the brain,
the light rays must land on the retinal cells in focus and with appropriate intensity.
The cornea, pupil (the center of the iris) and the lens are responsible for meeting
these requirements.
When light moves from one medium (such as air) into another medium
(such as the cornea or
lens), any rays not entering at a 90 degree angle will be refracted,
or bent. Because both the cornea and lens have curved surfaces, they
refract some of the
light rays entering the eye. In doing so, they compress the image of
what we see so that
a large amount of visual information can be processed by a small
amount of retinal
tissue. The cornea refracts more light than the lens does because its
surface is more curved, but the lens has the ability to change its
shape, and therefore
fine-tune the amount of refraction necessary to focus the light rays
on the retina. This
process is known as accommodation.
 The refraction of light rays as they pass from one medium to
another (a), such as through the cornea and lens (b). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The refraction of light rays as they pass from one medium to
another (a), such as through the cornea and lens (b). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The lens changes its shape in response to changes in tension of the ciliary muscles on
the suspensory ligaments (also called zonules) that hold the lens in place. When the ciliary muscles contract,
the suspensory ligaments are less taught, causing the lens to become slightly more
spherical and refract light more. This is what happens when objects that are being
viewed are close, or moved closer. Light coming from objects that are far away do not
require as much refraction and are viewed with the ciliary muscles relaxed and more
tension on the lens, which makes it more oblong. The relationship between the ciliary
muscles and the taughtness of the suspensory ligaments is a counterintuitive one for
most individuals, but the eye has a unique anatomy the leads to this relationship. See
the following video.
Ciliary Muscle Contraction
Using drawn models, the narrator explains the relationship between the ciliary
muscles and the taughtness of the suspensory ligaments.
 Accommodation of the lens with distant and near
vision. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Accommodation of the lens with distant and near
vision. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Along with accommodation of the lens when objects are near, the pupil also tends to
constrict to allow less peripheral light to enter the posterior chamber of the eye. In
doing so, objects can be viewed more crisply. The pupil will also constrict when
conditions are bright and dilate under low light conditions. This way the retina can
receive an appropriate amount of light to activate its photoreceptors without bleaching
them with too much light.
Sometimes the structures of the eye do not refract light appropriately, such that it
focuses either in front of (myopia) or behind (hyperopia) the retina. This can happen,
for instance, when the eye is not perfectly round. In order to correct for abnormalities
in light refraction, glasses or contact lenses can be added to the system to better
focus light on the retina and improve vision.
 Correcting abnormalities in light refraction in the eye. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Correcting abnormalities in light refraction in the eye. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Normal light refraction leads to the light rays converging on the retina (a). In the
case of hyperopia, the light rays focus behind the retina. This is corrected using a
convex lens to begin to bend the light before it reaches the cornea (b). In the case of
myopia, the light rays focus in front of the retina. This is corrected using a concave
lens to diverge the light rays before it reaches the cornea (c).
We have already discussed the structures of the eye that deliver and focus light on the
retina. The retina is composed of a several layers and contains specialized cells for
the initial processing of visual stimuli, with the rest of the visual processing
occuring in the central nervous system.
The photoreceptors are found in the retinal layer closest to the back of the eye
(outermost layer). When stimulated by light energy, they change their membrane potential
and alter the amount of neurotransmitter released onto the bipolar
cells. The bipolar cells connect to the retinal ganglion
cells (RGC) where amacrine cells also contribute to
retinal processing such as contrast enhancement and edge detection. The axons of RGCs,
which are lying at the innermost aspect of the retina, collect at the optic disc and leave the eye as the optic nerve. Because of the axons passing
through the wall of the eye at the optic disc, there are no photoreceptors resulting in
a “blind spot” in the retina. The blind spot in either retina falls in the medial retina
and does not process corresponding regions of the visual field.
 Layers of the retina in stained tissue (a) and as a drawing (b). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Layers of the retina in stained tissue (a) and as a drawing (b). This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
At the exact center of the retina is a point where light is focused by the lens and the
greatest visual acuity is found. This is known as the fovea and it is a small dimple in the layers of the retina where
there are no blood vessels, ganglion cells or bipolar cells to interrupt light reaching
the receptor cells. Because more light passes to the receptor cells at the
fovea, it is in this region that visual acuity is the greatest. From this central point
of the retina, visual acuity drops off towards the peripheral retina. This difference is
easily evidenced by looking directly at a word in the middle of this paragraph. The
visual stimulus exactly in the middle of the field of view falls on the fovea and is in
the sharpest focus. Without moving your eyes off that word, notice that words at the
beginning or end of the paragraph are not in focus. Beyond the words on your computer
screen, visual stimuli are less sharp to the point where the edges of vision have vague,
blurry shapes that cannot be clearly identified. A large part of neural function to
support the visual system is concerned with moving the eyes and head so that important
visual stimuli are centered on the fovea of the retina.
 Anatomy of the fovea. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomy of the fovea. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Light falling on the retina causes chemical changes to pigment molecules (called opsins) in photoreceptors, ultimately leading to a change in the
activity of the retinal ganglion cells. Photoreceptor cells have two parts, the inner segment and the outer segment (Figure
9).
 Structure of the photoreceptor cells. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The inner segment contains the nucleus and other common organelles of a cell while
the outer segment is a specialized region of the cell where photoreception takes place.
There are two types of photoreceptors, rods and cones, based on the shape of their outer segment. The rod-shaped outer
segments of rod photoreceptors contain a stack of membrane-bound discs that contain a
photosensitive opsin pigment called rhodopsin, which is sensitive
to a wide bandwidth of light (white light). The cone-shaped outer segments
of cone cells contain one of three photosensitive opsin pigments, called photopsins.
Each of the three photopsins are sensitive to a
particular bandwidth of light, corresponding to the colors of red,
green or blue, allowing for the ability to distinguish color.
Structure of the photoreceptor cells. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The inner segment contains the nucleus and other common organelles of a cell while
the outer segment is a specialized region of the cell where photoreception takes place.
There are two types of photoreceptors, rods and cones, based on the shape of their outer segment. The rod-shaped outer
segments of rod photoreceptors contain a stack of membrane-bound discs that contain a
photosensitive opsin pigment called rhodopsin, which is sensitive
to a wide bandwidth of light (white light). The cone-shaped outer segments
of cone cells contain one of three photosensitive opsin pigments, called photopsins.
Each of the three photopsins are sensitive to a
particular bandwidth of light, corresponding to the colors of red,
green or blue, allowing for the ability to distinguish color.
 Sensitivity of rod and cone photoreceptors to wavelengths of
light. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Sensitivity of rod and cone photoreceptors to wavelengths of
light. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
When a photoreceptor cell is activated by a photon near the wavelength it is sensitive
to, the energy from the light creates a change in its opsin molecule called photoisomerization. Photoisomerization is the first step in a
process that ultimately leads to a change in membrane potential of the photoreceptor.
Until the opsin is changed back to its original shape, the photoreceptor cell cannot
respond to light energy, which is called bleaching. When a large
group of opsins are bleached, vision will be affected until enough opsins can return to
the receptive state. You may have experienced this after the bright flash from a
camera.
Light and Dark Adaptation
Because rhodopsin found in the rod cells is most sensitive to white light while the cone
cells are color specific, rods are suited for vision in low-light conditions and cones
are suited for brighter conditions. In normal sunlight, rhodopsin will be constantly
bleached and the cones are active. In a darkened room, there is not enough light to
activate cone opsins, and vision is entirely dependent on rods. Rods are so sensitive to
light that a single photon can result in an action potential from the corresponding RGC.
The three cone photopsins, being sensitive to different wavelengths of light, can aid in
color vision. By comparing the activity of the three different cones, the brain can
extract color information from visual stimuli. Since rods are bleached when cones are
active and cones cannot react to low-intensity light, rods result in monochromatic
vision. In a dark room, everything appears as a shade of gray shadow. If you think that
you can see colors in the dark, it is most likely because your brain knows what color
something is and relies on that memory. If you are walking through your dark living room
and you are certain that the couch appears green, this is because you already know what
color it is, not because you perceive it with rod photoreceptors.
The photoreceptors, and other neuronal cells of the retina, send varied types of
information to the brain. These include light intensity, colors and the spatial
distribution of the information received. All of this information is then carried along
the optic nerve and into the optic tract to be distributed to nuclei in the brain. At
the point where the optic nerve becomes the optic tract, the optic chiasm is found. At
this point, fibers carrying information from the nasal half of the retina on each side
decussate (cross over), such that the information from the nasal half of the retina of
the left eye crosses over to the right side of the brain and vice versa. In doing so,
the left side of the brain receives information from the right visual field of each eye,
and the right side of the brain receives information from the left visual field of each
eye. This matches the sidedness of the brain to motor
control. For example, visual information from the left side of the body, and motor
control of the left limbs, are both processed by the right hemisphere of the brain.
 Depiction of how visual information has sidedness in the
brain. The diagram shows how information from the right visual field is
delivered to the left brain and how information from the left visual field is delivered
to the right side of the brain. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Depiction of how visual information has sidedness in the
brain. The diagram shows how information from the right visual field is
delivered to the left brain and how information from the left visual field is delivered
to the right side of the brain. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Visual information from the optic tract is sent to a variety of nuclei in the brain.
These nuclei, along with the type of processing they are involved in are summarized in
table below.
| Brain structures involved in visual processing. |
|---|
| Nuclei |
Role |
| Lateral geniculate nucleus of the thalamus |
Project to the occipital lobe for processing of visual perception |
| Superior colliculus |
Control of eye movements |
| Pretectum |
Pupillary light reflex |
| Suprachiasmatic nucleus of the hypothalamus |
Hormonal control |
|
The majority of the visual information flows through the lateral geniculate nucleus of
the thalamus into the occipital lobe for perception of vision. From here fibers will
carry some information to regions of the parietal and temporal lobes, called the visual
association areas. These areas contribute to object recognition (such as recognizing a
face) and motion processing (such as catching a moving ball).
Educational Video on The Vision System - Diopsys
A transverse section through the brain depicts the visual pathway from the eye
to the occipital cortex. The first half of the pathway is the projection from the
retinal ganglion cells through the optic nerve to the lateral geniculate nucleus in
the thalamus on either side. This first fiber in the pathway synapses on a thalamic
cell that then projects to the visual cortex in the occipital lobe where “seeing,”
or visual perception, takes place.
Observation...It is important to recognize when popular media and online sources oversimplify
complex physiological processes so that misunderstandings are not generated. This
video was created by a medical device manufacturer who might be trying to highlight
other aspects of the visual system than retinal processing. The statement they make
is not incorrect, it just bundles together several steps, which makes it sound like
RGCs are the traducers, rather than photoreceptors.
Hearing is the transduction of sound waves into a neural signal that relies on the
structures of the ear. The outwardly visible structure that is often referred
to as the ear is more correctly referred to as the outer ear
(external ear), or the auricle. The C-shaped
curves of the auricle direct sound waves towards the ear canal, which enters into the
skull through the external auditory meatus of the temporal bone. At the end of the ear
canal is the tympanic membrane, or ear drum, which vibrates with
the movement of air in sound waves.
 Anatomy of the Ear. The outer ear is
the auricle and ear canal through to the tympanic membrane. The middle ear contains
the ossicles and is connected to the pharynx by the auditory tube. The inner ear is
the cochlea and vestibule which are responsible for hearing and equilibrium,
respectively. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomy of the Ear. The outer ear is
the auricle and ear canal through to the tympanic membrane. The middle ear contains
the ossicles and is connected to the pharynx by the auditory tube. The inner ear is
the cochlea and vestibule which are responsible for hearing and equilibrium,
respectively. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Along the length of the ear canal are ceruminous glands that
contribute to the production of cerumen (earwax). Because cerumen
is sticky it can help prevent small particles from finding their way to the tympanic
membrane. Cerumen also helps prevent bacterial growth, waterproofs the auditory canal
and tympanic membrane, and may be a deterrent to small insects.
The middle ear consists of a space spanned by three small bones,
the ossicles, which amplify the movements of the tympanic
membrane. These small bones are the malleus, incus, and stapes, which are Latin names that roughly
translate to hammer, anvil, and stirrup. The malleus is attached to the tympanic
membrane and articulates with the incus, which articulates with the stapes. The stapes
is then attached to the inner ear where the sound waves will be
transduced to a neural signal.
The middle ear is also connected to the pharynx through the auditory
tube (Eustachian tube) that helps equilibrate air pressure across the tympanic
membrane. When flying, you may have experienced what happens when the pressures across
the tympanic membrane are not equal. As the plane climbs, pressure on the outside of the
membrane decreases. If there is not a corresponding decrease in pressure in the middle
ear, the pressure difference will cause the eardrum to push outward, causing pain and
muffled hearing. The auditory tube is normally closed, but will typically open when
muscles of the pharynx contract during swallowing or yawning. For this reason, chewing
gum or drinking as the plane climbs will often relieve these symptoms. The auditory tube
also provides a pathway of drainage for fluids that accumulate during middle ear
infections (otitis media). Unfortunately, it is also the auditory tubes that play a role
in causing otitis media, as microorganisms can use this path to move from the pharynx
into the middle ear. This is especially common in children.
The inner ear is entirely enclosed within the temporal bone. It has two
separate regions, the cochlea and vestibule,
which are responsible for hearing and balance, respectively. The neural signals from the
two regions of the inner ear are relayed to the brainstem through separate fiber
bundles, but which run together as the vestibulocochlear nerve.
Sound information is transmitted from the middle ear to the inner ear via the stapes
attachment to the oval window, which is a
membrane at the
beginning of the cochlea. As the tympanic membrane vibrates from
sound waves, the
ossicles amplify that vibration, and then the oval window moves with
the same vibrations. The oval window is at the beginning of a tube that
runs the length of the
cochlea to its tip (helicotrema) and back alongside itself to end
at another membrane called the round window (secondary tympanic
membrane).
 (a) Simplified Anatomy of the Cochlea. The cochlea can be
modeled as a long tube running from the oval window, out to the helicotrema, and back.
(b). Sound Wave Transmitted into the Cochlea. As sound is
transmitted from air to the cochlea through the oval window, it creates a wave within
the fluid of the cochlea (often called a standing wave). This wave creates displacement
in the membranes of the cochlear duct, where sound is sensed. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
(a) Simplified Anatomy of the Cochlea. The cochlea can be
modeled as a long tube running from the oval window, out to the helicotrema, and back.
(b). Sound Wave Transmitted into the Cochlea. As sound is
transmitted from air to the cochlea through the oval window, it creates a wave within
the fluid of the cochlea (often called a standing wave). This wave creates displacement
in the membranes of the cochlear duct, where sound is sensed. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
As the oval window is pushed in by sound waves, fluid within this
tube is pushed along its length and the round window at its other end can bulge out as a
result of that movement. Likewise, when the oval window is pulled back, the fluid inside
this tube is drawn back and the round window can pucker in to compensate. As vibrations
of the tympanic membrane are transmitted through the ossicles, a wave (often referred to
as standing wave because of its properties) is created within the fluid in the cochlea that displaces sections of the cochlear partition (cochlear duct and basilar membrane). It is
these waves that are detected by the sensing cells found attached to the basilar
membrane.
The tube running from the oval to the round window in the cochlea is separated into two
spaces. From the oval window to the tip of the cochlea the tube is
referred to as the scala vestibuli and from the tip of the cochlea
back to the round window it is the scala tympani. These spaces can
be seen in a cross-section of one turn of the cochlea.
 Anatomy of the Cochlea. The cochlea is a spiral structure
(a) divided into three chambers (b). The middle chamber, the cochlear duct, contains the
spiral organ that has hair cells (c) for sensing the vibrations we perceive as
sound. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomy of the Cochlea. The cochlea is a spiral structure
(a) divided into three chambers (b). The middle chamber, the cochlear duct, contains the
spiral organ that has hair cells (c) for sensing the vibrations we perceive as
sound. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The two spaces are on
either side of the cochlear duct, which is the space that contains
the structures that transduce sound into the neural signal. Those structures are
contained within the spiral organ or organ of
Corti, which lies on top of the basilar membrane that
separates it from the scala tympani. The spiral organ contains hair
cells with stereocilia on their apical membrane. The stereocilia bend in response to movement of the basilar membrane relative to
the partially fixed tectorial membrane. Depending on which
direction the stereocilia bend, they open or close ion channels, leading to signal
changes in the cochlear nerve.
As a standing wave is set up within the scala vestibuli and scala tympani in response to
movement at the oval window, the basilar membrane responds by moving at a specific spot,
dependent on the frequency of the standing wave.
 Frequency Response of the Cochlea. Different frequencies
are sensed in different regions of the cochlea. High frequencies (high pitch) are sensed
near the base of the cochlea, whereas low frequencies are sensed near the tip of the
cochlea. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Frequency Response of the Cochlea. Different frequencies
are sensed in different regions of the cochlea. High frequencies (high pitch) are sensed
near the base of the cochlea, whereas low frequencies are sensed near the tip of the
cochlea. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The cochlea encodes auditory
stimuli based on frequency between 20 Hz and 20,000 Hz, the range of human hearing.
Higher frequencies (higher pitch) cause the basilar membrane close to the base of the
cochlea to move and lower frequencies (lower pitch) cause the basilar membrane closer to
the tip of the cochlea to move. Loudness, or sound intensity, is determined by the
number of hair cells in an area that are stimulated. A louder sound produces greater
displacement of the basilar membrane, leading to a greater number of stereocilia
responding.
The information sensed by the spiral organ of the cochlea is transmitted into the brain
via the vestibulocochlear nerve (cranial nerve VIII) to nuclei in the pons. At the level
of the pons and related connections in the midbrain, some processing of auditory
information occurs, including identifying where a sound is coming from and responding to
loud noises. From the pons and midbrain, fibers carrying auditory information project to
the thalamus and then, from there to the primary auditory cortex of the temporal lobe.
It is here that the conscious perception of sound is located.
Along with hearing, the inner ear is responsible for encoding information about
equilibrium (the sense of balance), which it does in the vestibular apparatus.
 Structures of the Vestibular Apparatus. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Structures of the Vestibular Apparatus. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Similar to the cochlea, the vestibular structures use hair cells with stereocilia to
detect movement of fluid, in this case, in response to changes in head position or
acceleration. Detection of head position when the body is stationary is termed static equilibrium. The information for static equilibrium comes
from the utricle and saccule. Dynamic equilibrium is the perception of acceleration. Information
for dynamic equilibrium can come from the utricle and saccule, which detect linear
acceleration, and/or the semicircular canals, which detect angular acceleration. The
neural signals generated from the vestibule are transmitted to the brainstem and
cerebellum from sensory neurons in the vestibular ganglion.
The saccule and utricle each contain a sense organ, called the macula, where stereocilia and their supporting cells are found. These maculae
(plural) are oriented 90 degrees to one another so that they respond to positions in
different planes.
 Structure of the Maculae. The macula utriculi (macula of
the utricle) lies horizontally while the macula sacculi lies vertically (a). If the head
is tilted, the dense otolithic membrane will cause the stereocilia of the hair cells to
move from the straight position (b) to the bent position (c), sending signals to the
central nervous system that the head has been tilted forward. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The organs can respond to changes in position and
acceleration because the tips of their stereocilia project into a dense otolithic membrane made up of a mixture containing granules of calcium and
protein, called otoliths, which gives rise to their common name,
the otolithic organs. When the maculae (otolithic organs) move,
gravity causes the dense otolithic membrane to move relative to the less dense cell
layer beneath the stereocilia. This causes the stereocilia to bend, initiating action
potentials in the vestibular nerve fibers that innervate them.
Structure of the Maculae. The macula utriculi (macula of
the utricle) lies horizontally while the macula sacculi lies vertically (a). If the head
is tilted, the dense otolithic membrane will cause the stereocilia of the hair cells to
move from the straight position (b) to the bent position (c), sending signals to the
central nervous system that the head has been tilted forward. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The organs can respond to changes in position and
acceleration because the tips of their stereocilia project into a dense otolithic membrane made up of a mixture containing granules of calcium and
protein, called otoliths, which gives rise to their common name,
the otolithic organs. When the maculae (otolithic organs) move,
gravity causes the dense otolithic membrane to move relative to the less dense cell
layer beneath the stereocilia. This causes the stereocilia to bend, initiating action
potentials in the vestibular nerve fibers that innervate them.
Bundles of stereocilia are arranged in various directions, so that any direction of
inclination will depolarize a subset of the hair cells and hyperpolarize a corresponding
subset of sensory neurons. How the body senses head position and the linear (horizontal or vertical)
direction of acceleration is determined by the specific pattern of hair-cell activity
across the maculae.
The semicircular canals are three ring-like extensions from the vestibule (the region
containing the saccule and utricle). One is oriented in the horizontal
plane and two are in the vertical plane. The vertical canals are 45o off of
the sagittal plane, one is anterior and one is posterior.
 Structure and Function of the Semicircular Canals. The
three canals each have an ampulla containing a crista ampullaris and cupula (a). When
the head is stationary, the cupula, and embedded stereocilia, are not bent (b). When the
head rotates in the same plane as one of the canals, the fluid in the canal (endolymph)
lags, leading to bending of the stereocilia in the cupula, which initiates nerve
impulses. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Structure and Function of the Semicircular Canals. The
three canals each have an ampulla containing a crista ampullaris and cupula (a). When
the head is stationary, the cupula, and embedded stereocilia, are not bent (b). When the
head rotates in the same plane as one of the canals, the fluid in the canal (endolymph)
lags, leading to bending of the stereocilia in the cupula, which initiates nerve
impulses. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
At the base of each
semicircular canal, where it meets with the vestibule is an enlarged region known as the
ampulla, which contains a hair-cell containing structure,
called the crista ampullaris that responds to rotational movement.
The stereocilia of the hair cells extend into the cupula, a
membrane that attaches to the top of the ampulla.
When the head rotates in a plane parallel to the semicircular canal, the fluid in the
canal does not move as quickly as the head is moving. This pushes the cupula in the
opposite direction, deflecting the stereocilia and creating a nerve impulse. Considering
the semicircular canals on either side of the head, three orthogonal planes are defined,
the horizontal plane with both horizontal canals, and two vertical planes 90o
to each other with the anterior canal from one side and the posterior canal from
the other. In each pair, deflection of the cupula on one side of the body causes
depolarization of the hair cells while the same movement causes hyperpolarization of the
hair cells on the other side of the body. For example, when the head rotates to the
right, the horizontal canals are active and the right side depolarizes while the left
hyperpolarizes, indicating the direction of the movement. By comparing the relative
movements of all six semicircular canals, the vestibular system can establish movement
in any direction within three-dimensional space.
Information from the vestibular apparatus projects to a wide range of structures in the
brain. The information enters the brain via the vestibulocochlear nerve (cranial nerve
VIII) where it synapses at vestibular nuclei in the pons and medulla. From here,
information travels to a number of other regions to stimulate reflexes and develop our
conscious awareness of position and movement.
Taste, or gustation, is a sense that develops
through the interaction of dissolved molecules with taste buds. Currently five
sub-modalities (tastes) are recognized, including sweet, salty, bitter, sour, and umami (savory taste or the taste of protein). Umami is the most
recent taste sensation described, gaining acceptance in the 1980s. Further research has
the potential to discover more sub-modalities in this area, with some scientists
suggesting that a taste receptor for fats is likely.
Taste is associated mainly with the tongue, although there are taste (gustatory)
receptors on the palate and epiglottis as well. The surface of the tongue, along with
the rest of the oral cavity, is lined by a stratified squamous epithelium. In the
surface of the tongue are raised bumps, called papilla, that
contain the taste buds. There are three types of papilla, based on their appearance: vallate, foliate, and fungiform.
 Structures Associated with Taste. The tongue is covered
with papillae (a), which contain taste buds (b and c). Within the taste buds are
specialized taste cells (d) that respond to chemical stimuli dissolved in the saliva
and, in turn, activate sensory nerve fibers in the facial and glossopharyngeal
nerves. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Structures Associated with Taste. The tongue is covered
with papillae (a), which contain taste buds (b and c). Within the taste buds are
specialized taste cells (d) that respond to chemical stimuli dissolved in the saliva
and, in turn, activate sensory nerve fibers in the facial and glossopharyngeal
nerves. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
The number of taste buds within papillae
varies, with each bud containing several specialized taste cells
(gustatory receptor cells) for the transduction of taste stimuli.
These receptor cells release neurotransmitters when certain chemicals in ingested
substances (such as food) are carried to their surface in saliva. Neurotransmitter from
the gustatory cells can activate the sensory neurons in the facial and glossopharyngeal
cranial nerves.
As previously mentioned, five different taste sensations are currently recognized. The
first, salty, is simply the sense of Na+ concentration in the saliva. As the
Na+ concentration becomes high outside the taste cells, a strong
concentration gradient drives their diffusion into the cells. This depolarizes the
cells, leading them to release neurotransmitter.
The sour taste is transduced similar to that of salty, except that it is a response to
the H+ concentration released from acidic substances (those with low pH),
instead of a response to Na+. For example, orange juice, which contains
citric acid, will taste sour because it has a pH value of about 3. Of course, it is
often sweetened so that the sour taste is masked. As the concentration of the hydrogen
ions increases because of ingesting acidic compounds, the depolarization of specific
taste cells increases.
The other three tastes; sweet, bitter and umami are transduced through G-protein coupled
cell surface receptors instead of the direct diffusion of ions like we discussed with
salty and sour. The sweet taste is the sensitivity of taste cells to the presence of
glucose dissolved in the saliva. Molecules that are similar in structure to glucose will
have a similar effect on the sensation of sweetness. Other monosaccharides such as
fructose or artificial sweeteners like aspartame (Nutrasweet™), saccharine, or sucralose
(Splenda™) will activate the sweet receptors as well. The affinity for each of these
molecules varies, and some will taste “sweeter” than glucose because they bind to the
G-protein coupled receptor differently.
The bitter taste can be stimulated by a large number of molecules collectively known as
alkaloids. Alkaloids are essentially the opposite of acids,
they contain basic (in the sense of pH) nitrogen atoms within their structures. Most
alkaloids originate from plant sources, with common examples being hops (in beer),
tannins (in wine), tea, aspirin, and similar molecules. Coffee contains alkaloids and is
slightly acidic, with the alkaloids contributing the bitter taste to coffee. When enough
alkaloids are contained in a substance it can stimulate the gag reflex. This is a
protective mechanism because alkaloids are often produced by plants as a toxin to deter
infectious microorganisms and plant eating animals. Such molecules may be toxic to
animals as well, so we tend to avoid eating bitter foods. When we do eat bitter foods,
they are often combined with a sweet component to make them more palatable (cream and
sugar in coffee, for example).
The taste known as umami is often referred to as the savory taste. The name was created
by the Japanese researcher who originally described it. Like sweet and bitter, it is
based on the activation of G-protein coupled receptors, in this case by amino acids,
especially glutamine. Thus, umami might be considered the taste of proteins, and is most
associated with meat containing dishes.
Once the taste cells are activated by molecules liberated from the things we ingest, they
release neurotransmitters onto the dendrites of sensory neurons. These neurons are part
of the facial and glossopharyngeal cranial nerves, as well as a component within the
vagus nerve dedicated to the gag reflex. The facial nerve connects to taste buds in the
anterior third of the tongue. The glossopharyngeal nerve connects to taste buds in the
posterior two thirds of the tongue. The vagus nerve connects to taste buds in the
extreme posterior of the tongue, verging on the pharynx, which are more sensitive to
noxious stimuli like bitterness.
Axons from the three cranial nerves carrying taste information travel to the medulla.
From there much of the information is carried to the thalamus and then routed to the
primary gustatory cortex, located near the inferior margin of the post-central gyrus. It
is the primary gustatory cortex that is responsible for our sensations of taste. And,
although this region receives significant input from taste buds, it is likely that it
also receives information about the smell and texture of food, all contributing to our
overall taste experience. The nuclei in the medulla also send projections to the
hypothalamus and amygdalae, which are involved in autonomic reflexes such as gagging and
salivation.
The other special sense responsive to chemical stimuli is the sense of the smell, or
olfaction. The olfactory receptor neurons are incorporated into a limited region of the
nasal epithelium in the superior nasal cavity.
 Anatomy of the Structures Involved in Smell (Olfaction).
The olfactory bulb (1) contains mitral cells (2) that receive information from the
olfactory cells (6). The olfactory cells are found within the nasal epithelium (4) and
pass their information through the cribriform plate (3) of the ethmoid bone. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Anatomy of the Structures Involved in Smell (Olfaction).
The olfactory bulb (1) contains mitral cells (2) that receive information from the
olfactory cells (6). The olfactory cells are found within the nasal epithelium (4) and
pass their information through the cribriform plate (3) of the ethmoid bone. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
This region is referred to as
the olfactory epithelium and contains bipolar sensory neurons with
dendrites extending from the apical surface of the epithelium into the mucus lining the
nasal cavity. As airborne molecules are inhaled through the nose, they pass over the
olfactory epithelium and dissolve into the mucus. The odorant
molecules bind to proteins that keep them dissolved in the mucus and help transport
them to the olfactory dendrites. The odorant-protein complex binds to a receptor protein
on the membrane of the olfactory cell. The olfactory odorant receptors are G-protein
coupled receptors that will cause a transient depolarization in membrane potential that
will lead to an action potential if the stimulus is strong enough.
The axons of the olfactory neurons extend from the basal surface of the epithelium,
through an olfactory foramen in the cribriform plate of the ethmoid bone, and into the
olfactory bulb, located on the ventral surface of the frontal lobe. Collectively the
axons that pass through the cribriform plate are called the olfactory
nerve. From the olfactory bulb, axons project to structures within the limbic
cortex, including the cortex in the temporal lobe associated with long-term memory
formation.
 Central Nervous System Regions that Receive Information from
the Olfactory Bulb. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Central Nervous System Regions that Receive Information from
the Olfactory Bulb. This work by Cenveo is licensed under a
Creative Commons Attribution 3.0 United States (http://creativecommons.org/licenses/by/3.0/us/).
Smell is the one sensory modality that does not require a synaptic
connection in the thalamus before connecting to the cerebral cortex. Smell can often be
a potent trigger for memories because of this intimate connection of the olfactory
system with the cerebral cortex. It can also trigger visceral reflexes through
connections within the reticular formation. For example, some people will vomit at the
smell of another person’s vomit.
Because of the noxious chemicals that are inhaled into the nasal cavity, these cells need
to be replaced on a regular basis. The nasal epithelium, including the olfactory cells,
can be harmed by airborne toxic chemicals. To reduce the risk of olfactory neurons being
harmed by these toxins, they have a limited lifespan of about 60 days. Because of this
imposed cell death, stem cells within the nasal epithelial layer differentiate into new
olfactory cells. In doing so, axons of the new neurons have to find their way to the
targets in the olfactory bulb. They are able to do this by growing along the axons that
are already in place in the cranial nerve. This is the only place in the body where we
continually make new neuronal cells.
If any event, such as blunt force trauma of a car accident, leads to the loss of the
olfactory nerve, the sense of smell can be lost. This condition is known as anosmia. One way this condition can develop is if the brain moves
relative to the ethmoid bone, which is likely to shear (tear) the axons of the olfactory
cells. Professional fighters can have anosmia as a result repeated trauma to the head.
Certain pharmaceuticals, such as antibiotics, can cause anosmia because they can kill
off all the olfactory neurons at once. If no axons are in place within the olfactory
nerve, then the axons cannot grow back into the olfactory bulb leaving the person with a
long-term loss of smell. There are temporary causes of anosmia, as well, usually based
on inflammatory responses in relation to infection or allergy.
You have probably noticed that when you have a cold, the associated loss of smell usually
results in food tasting bland. Since taste is comprised of only a small number of
sensations, we use the olfactory system to recognize differences between many foods,
especially in terms of the spices used. If we lack the numerous smells to enrich the
taste experience, we often add extra seasonings to further stimulate the tongue.
The ability of olfactory neurons to be replaced by stem cells can be lost with age
leading to age-related anosmia. When this affects an elderly person, they often turn to
using increased salt on their food. Unfortunately, increased sodium intake also leads to
an increase in blood volume and blood pressure.
Before taking the quiz below, consider again the learning objectives for this unit. Could
you demonstrate each of these objectives? If so, you will be ready for the assessment
below. If not, consider reviewing content related to these objectives before attempting
the assessment.
- Classify receptors based on structure, location relative to the stimulus, and types of signals they transduce.
- Explain the distribution of receptors involved in providing information for our general (somesthetic) senses.
- Describe the types of information (modality) detected by the
receptors associated with the somesthetic senses and the phenomenon of
adaptation.
- Explain pain function, nociceptor distribution, and distinguish the fiber types that carry their signals.
- Describe pain in terms of hyperalgesia, analgesia, and receptive field.
- Define dermatome and explain how dermatomes can be used in neurological exams for diagnosing nerve damage.
- Describe reflexes, reflex repsonses, and distinguish types of reflexes.
- Identify and describe the functions of the accessory eye structures, the tunics, and the optical components of the eye.
- Identify the muscles that help to coordinate eye movement.
- Describe the structures and functions of the eye.
- Explain how the path of light through the eye causes vision.
- Trace the path of nerve impulses from the retina to various parts of the brain.
- Identify the hearing structures of the outer, middle and inner ear and describe their functions.
- Describe how the various structures of the ear conduct and transduce sound.
- Describe the path of nerve impulses from the ear to various parts of the brain.
- Distinguish between static and dynamic equilibrium, describe the structures involved, and their functions.
- Explain the gustation and describe the structures involved.
- Describe the path of nerve impulses from the gustatory receptors to various parts of the brain.
- Explain how odorants activate olfactory receptors.
- Describe the path of nerve impulses from the olfactory receptors to various parts of the brain.
- Predict the types of problems that would occur in the body if the olfactory system was not functioning normally.
In the introductory units we covered the major themes of
anatomy and physiology, and we have addressed these themes as they
relate to each specific organ system. In this review and synthesis unit
we will present specific examples of how these themes relate to every
aspect of physiology. We will present examples specific to different
organ system units to review both the major themes and your
understanding of the different organ systems.
In this unit we will also examine pathological conditions
related to anatomy and physiology focusing on two conditions: sickle
cell anemia and obesity. Sickle cell anemia is a genetic disease that
impacts specific populations. This condition is present at birth and is
present throughout the individual’s lifetime. On the other side of the
spectrum, is obesity. Although there are some genetic factors which
influence obesity, usually environmental and psychological factors are
as much to blame for the pathologies associated with obesity.
Through these examples of physiology and pathology, we will
examine anatomy and physiology as it relates to: Structure and function,
levels of organization, homeostasis, and integration of systems.
As you have seen, function relies completely upon structure and
altered structure can greatly affect function. The following exercises
take a look at one way this relationship plays out in the Urinary
system.
Example
The Bladder
Within the urinary system, recall that the bladder is the organ that
stores urine until elimination by urination. Since urination production
is continuous and elimination occurs less frequently, the amount stored
changes over time. We will examine how the anatomical structure of the
bladder allows its physiological function.
To permit large volume changes, the lining of the urinary bladder has rugae, similar to the lining of the digestive system.
The detrusor muscle in the urinary bladder contains multiple layers
of smooth muscle cells that align in different directions. These muscles
are aligned in the long axis, as well as around the bladder as circles
and as spirals. These smooth muscles are able to stretch and signal that
voiding is necessary.
Example
Layers of the Epidermis
There are multiple layers of cells in the epidermis of the
integumentary system. The function of the collective structural layers
is to provide a continuous barrier that also renews. Within the
epidermis, each distinct layer has different structures and functions
related to their structure.
Example
Gastric Bypass
Obesity is usually treated with altered lifestyle changes including
altered diet and increased exercise. Occasionally, weight loss
medication is used to supplement lifestyle changes. However, in severe
cases, altering the stomach’s structure can also be used to reduce food
intake. Two major types of surgery include adjustable gastric band
(Lap-Band) and gastric bypass.
Gastric banding physically restricts the stomach from expanding.
Without the band, an adult’s stomach can expand from 50 mL to hold about
1 liter of food, and can distend to hold as much as 4 liters. There are
numerous structures which allow this distension function.
Less food can fit into a stomach with a gastric band, and individuals
feel full sooner. With gastric bypass surgery, stapling the stomach
reduces stomach volume. Also, a portion of the small intestine is
bypassed.
Example
Sickle Cell: Altered Red Blood Cell Shape
In sickle cell anemia, low oxygen conditions lead to an altered red
blood cell shape and reduced elasticity. Stiffer red blood cells can
lead to vessel occlusion and ultimately tissue damage.
As we’ve seen throughout this course, the human body is a
complex, hierarchical system. We’ve studied each level separately and
how each level builds to form, support and become a system. Let’s now
take a closer look at how these levels of organizations integrate.
Example
Electrical Conductivity in the Heart
We can only live for a few minutes without the heart beating. This
event, which occurs millions of times in a lifetime, is a function of
many length scales within the cardiac tissue of the heart.
Example
Oxygen for Cell Metabolism
Obtaining oxygen is important for metabolism in every cell throughout
the body. The respiratory system is dedicated to this exchange with the
environment. Coordination of respiratory and cardiovascular function
allows uptake of oxygen by respiration, exchange to the cardiovascular
system, and distribution of oxygen through the body by the
cardiovascular system.
Example
Sickle Cell's Gene Mutation Across Length Scales
In sickle cell anemia, there is a mutation in gene for the β-globin
protein, which is part of hemoglobin. The altered structure of β-globin
causes an aggregation of the proteins. When these proteins aggregate,
they distort the shape of the red blood cell and reduces red blood cell
elasticity. The abnormal cells get stuck in capillaries, reducing blood
flow to tissues and organs. Organ damage may result, permanently
affecting body function. Thus, a seemingly tiny error in DNA causes
significant changes in the body’s systems at higher levels.
Example
Obesity Followed Through the Levels of Organization
Obesity is caused by excessive food intake and reduced physical
activity. Genetics and cultural surroundings can also play a part. Let’s
examine how levels of organization all have contributing factors on
obesity:
Increased carbohydrates and lipid intake: at the molecular level,
excessive food intake can lead to accumulation of fat and altered body
chemistry.
Decreased activity leads to reduced muscle mass: at the cellular and
tissue level the inactivity of obesity is associated with decreased
activity, muscle tone and muscle mass.
Obesity is also strongly correlated with the local population demographics.
Red Blood Cells in Sickle Cell Anemia
Sickle cell anemia can lead to reduced oxygen delivery to
tissues. Also, reduced red blood cell elasticity can lead to cell
rupture and anemia. Anaemia is measured by a reduction in iron. It can
be caused by iron deficiency, insufficient red cell production or red
blood cell dysfunction; in this case dysfunction. When red cells are
sickled, they only have a lifespan of 10-20 days whereas normal red
blood cells have a lifespan of >100 days.
If red blood cells are not able to be replenished and the
patient becomes anemic then different regions of the body can be
deprived of oxygen. The effects of anemia can be compounded by
obstruction of blood vessels by sickled cells. When tissues do not have
sufficient oxygen, cells die by apoptosis or necrosis. Apoptosis is
programmed death, which is a natural part of remodeling: when tissues
are remodeled cells invade or proliferate (divide) to perform a function
and they are done the apoptose. This type of cell death does not cause
disruption to the cells surrounding the dying cell. Necrosis on the
other hand triggers uncontrolled release of cell products into
intracellular space which in turn initiates an inflammatory response in
the surrounding tissue.
Example
pH Balance
The lungs and kidneys work together to regulate the hydrogen ion
concentration (pH) of arterial blood. This pH must be kept within a
narrow range (7.38 - 7.42). An increase in pH (alkalosis) or decrease in
pH (acidosis) can result in profound effects within the body. For
example, certain amino acids are sensitive to the pH in their
environment. An unusual pH could result in conformational changes in
proteins and subsequently a change in the proteins’ functions.
Example
Blood Glucose
Solute and water balance is maintained when solutes and water enter
and exit the plasma at the same rate. When one material enters or exits
the plasma faster than the other, a change in volume and/or composition
occurs. The plasma concentrations of many substances are controlled by
specific regulatory mechanisms.
Example
Exercise
Body muscles use the energy from ATP molecules to generate the force
necessary to contract. A byproduct of this energy release is heat
release. This results in an increase in body temperature during
intensive exercise. The body compensates for extra heat by causing
vasodilation of blood vessels near the skin and by causing sweat glands
to release sweat.
Throughout the course, we considered organ systems as separate
systems. However, since the body functions as a single unit, the
physiology of organ systems cannot truly be understood in isolation of
other systems. Here, we will consider pathological conditions which
impact multiple organ systems. We will also highlight how a thorough
knowledge of physiology will allow a proper diagnosis of a disease of
one organ system caused by another.
Example
Diabeties
A podiatrist sees unhealed sores on a patient's feet. He inquires if
the patient has noticed any problems with vision in the past few months.
When the patient says that his eyes have been bad, the podiatrist
refers the patient to an endocrine specialist to be checked for
diabetes.
Example
Lymphatic: Splenic Sequestration Crisis
One of the main functions of the sinuses of the spleen is to break
open senescent (old) red blood cells for processing. In indiviudals with
severe sickle cell anemia, the spleen can be affected and vessels
become occluded. Splenic sequestration crises are painful enlargements
of the spleen, which causes nausea and abdomen bloating. This condition
can cause circulatory failure and be fatal if not treated.
Now, let's look at the condition of obesity in more depth. Below is
an activity that provides several sections of content and questions
that will help you recall, organize and apply what you have learned in
this course to the comprehensive condition of obesity.
Explore the following activity to learn how obesity affects each
system of the body.
The activity consists of three sections.
- The top left panel contains an image with hotspots. Use this for
navigation. Each hotspot connects to a section of unique material and
activities.
- The top right panel changes each time you choose a new section to
work through. Look to this panel for the information you will need to
solve the problems and questions that also appear for that section.
- The bottom panel changes each time you choose a new section as
well. You will find activities and questions here that are unique to
that section.
Be sure to click on each hotspot to ensure you complete all of the
questions within this activity. When you are sure you have finished each
section, complete the follow-up activity directly below this one.
Effects of Obesity on the Human Body
Introduction
Obesity Introduction
A woman comes for her annual check-up and is told that she is
obese. Her physician strongly urges her to lose weight as obesity is
very bad for her overall health.
Obesity is a condition that affects much of the body - both in ways we can and cannot see.
Directions: Explore common changes and results that
come from obesity by clicking on hotspots found on the line drawing in
the left panel. Clicking the questions tab on your right will bring up
questions about how this condition is related to other conditions.
Skin Imbalance
Numerous complications in the integumentary system can arise
as a result of obesity. For example, obesity can result in insulin
resistance, which subsequently leads to larger concentrations of insulin
circulating in the bloodstream. Excess insulin impacts the skin causing
abnormal growth, referred to as as hyperplasia.The hyperplasia can lead
to a disease known as acanthosis nigricans, which presents itself as
brown or black hyperpigmentation of the skin.
Increased strain on leg veins due to obesity can result in
fluid retention and swelling (edema), superficial capillary rupture, and
ulcers. The increased weight can also result in corns and calluses on
the feet. Most of these conditions can be resolved weight loss.
Sleep Apnea and Shallow Breathing
Obesity can hinder respiratory function. With fat
accumulation around the waste, the diaphragm muscle becomes distended.
The diaphragm is the primary inspiratory muscle for respiration. If the
diaphragm's function is impeded, muscles in the upper chest and throat
have more of the mechanical burden for breath.
Sleep apnea is a sleep disorder that presents itself with
abnormal pauses in breathing (known as apneas) that can last anywhere
from several seconds to a minute. The most common form of sleep apnea, obstructive sleep apnea,
can be caused or exacerbated by obesity. The low muscle-tone and
excessive amount of soft tissue in the throat area can cause breathing
to be obstructed. This subsequently results in temporary episodes of an
inability to breath.
Acid Reflux
Strong acids and enzymes (such as HCl and pepsin,
respectively) are generated by the stomach to promote food digestion.
The esophagus is protected by this gastric juice by the lower esophageal
sphincter, which holds shut the barrier between the bottom of the
esophagus and the top of the stomach. When stomach acid manages to make
its way through a faulty lower esophageal sphincter and into the
esophagus, it results in a condition known as acid reflux.
This phenomenon is colloquially known as heartburn because of the
burning sensation felt in the general area where the heart is located.
An adult stomach is designed to expand to allow the intake of
food. However, when a person habitually takes in large meals, the
stomach grows. In addition to stomach distension, the peritoneal cavity
has can gain increased visceral fat. This generally occurs as a result
of excess caloric intake.
Heart Strain
Obesity results in an increase in body fat and subsequently
body mass. This forces the heart to work harder and exert greater force
when pumping blood throughout the body. The increase in body fat also
results in greater levels of cholesterol in the blood vessels. Blood
vessel constriction further increases the strain on the heart. As the
heart is forced to work harder and harder, it requires greater
quantities of oxygen. Heart failure can result when lack of oxygen
results in death of cardiac muscle. This is typically a result of
impeded blood flow due to fat-clogged blood vessels.
Breast and/or Other Cancers. . .
Obesity has been found to be correlated with an increased
risk of breast cancer. This is speculated to be a result of increased
levels of estrogen in obese women. After menopause, the ovaries stop
producing hormones, which puts the burden of estrogen production on fat
tissue. Since obese women have greater amounts of fat tissue, their
estrogen levels are higher. This subsequently results in more rapid
growth of estrogen-responsive breast tumors.
Obesity is also associated with an increased risk of cancer
in the esophagus, pancreas, kidneys, thyroid, gallbladder, endometrium,
colon and rectum.
Urinary Incontinence
Urinary incontinence is the inability to control urine flow.
Obesity often presents itself with urinary incontinence because of
increased weight in the midsection. This causes extra pressure that
makes it more likely that the bladder will leak. This results in stress
incontinence, or the tendency for the bladder to leak when performing
everyday actions such as laughing or coughing. Obesity is also strongly
correlated with diabetes, which can cause damage to the nerves that
control bladder muscles.
Joint Strain
Joints are found at the location where two or more bones make
contact. Their general purpose is to allow movement, and they also
serve to provide mechanical support. Joints essentially carry the weight
of our bodies. So, the more weight they are forced to carry, the
greater the strain it places on the joints. Small changes in weight can
have substantial effects on joint forces in the hips and knees. Research
has shown that obese individuals are more likely to develop arthritis.
Poor Circulation
Calf muscles rely on a constant supply of fresh blood to the
muscle tissues. In a healthy individual, this blood is returned back up
to the body against gravity via a pumping system formed by the leg
muscles. The main driving force for this pumping system is supplied by
the calf muscles as they contract, which takes pressure off the veins,
allowing blood to return to the upper body. As blood passes through
these veins, one way valves found in the veins shut to prevent blood
from returning to the feet. This used blood is subsequently displaced by
the same volume of fresh blood.
Because obese individuals tend to lead a life of little
movement and activity, the ability of their legs to contribute to
circulation decreases substantially. This results in a disruption of the
aforementioned cycle, and used blood remains in the lower legs. This
causes water from lymph fluid to separate from the blood and fill spaces
in tissues, resulting in edema.